Efficacy
Efficacy
DELSTRIGO® (Doravirine/Lamivudine/tenofovir disoproxil fumarate)
Prescribing Information (United Kingdom) [External link]
PIFELTRO® (doravirine)
Prescribing Information (United Kingdom) [External link]
Doravirine/3TC/TDF = DELSTRIGO (doravirine/lamivudine/tenofovir disoproxil fumarate).
Licensed indication
PIFELTRO® (doravirine) 100 mg film-coated tablet is indicated, in combination with other antiretroviral medicinal products, for the treatment of adults, and adolescents aged 12 years and older, weighing at least 35 kg, infected with HIV-1, without past or present evidence of resistance to the NNRTI class.
DELSTRIGO® (100 mg doravirine/300 mg lamivudine/300 mg tenofovir disoproxil fumarate, equivalent to 245 mg tenofovir disoproxil) is indicated for the treatment of adults infected with HIV-1 without past or present evidence of resistance to the NNRTI class, lamivudine or tenofovir.
DELSTRIGO® is also indicated for the treatment of adolescents aged 12 years and older weighing at least 35 kg, who are infected with HIV-1, without the past or present evidence of resistance to the NNRTI class, lamivudine, or tenofovir and who have experienced toxicities which preclude the use of other regimens that do not contain tenofovir disoproxil.
Please consult the SmPC for further information before making any prescribing decisions.

Using FDA snapshot approach:
Participants in the DRIVE SHIFT study switched from a baseline regimen consisting of 2 NRTIs in combination with a PI plus either ritonavir or cobicistat, or elvitegravir plus cobicistat, or an NNRTI1
Virologic suppression was maintained for over 2 years in adults with HIV-1 who switched to DOR/3TC/TDF (DELSTRIGO) from a boosted PI, boosted elvitegravir, or an NNRTI2
Primary efficacy outcome
- The proportion of participants with HIV-1 RNA <50 copies/ml at Week 48 in the immediate switch group compared to Week 24 in the delayed switch group.
- The study demonstrated switching to DOR/3TC/TDF is non-inferior to continuing the baseline regimen for 24 weeks.
Virologic outcomes after switch to DOR/3TC/TDF
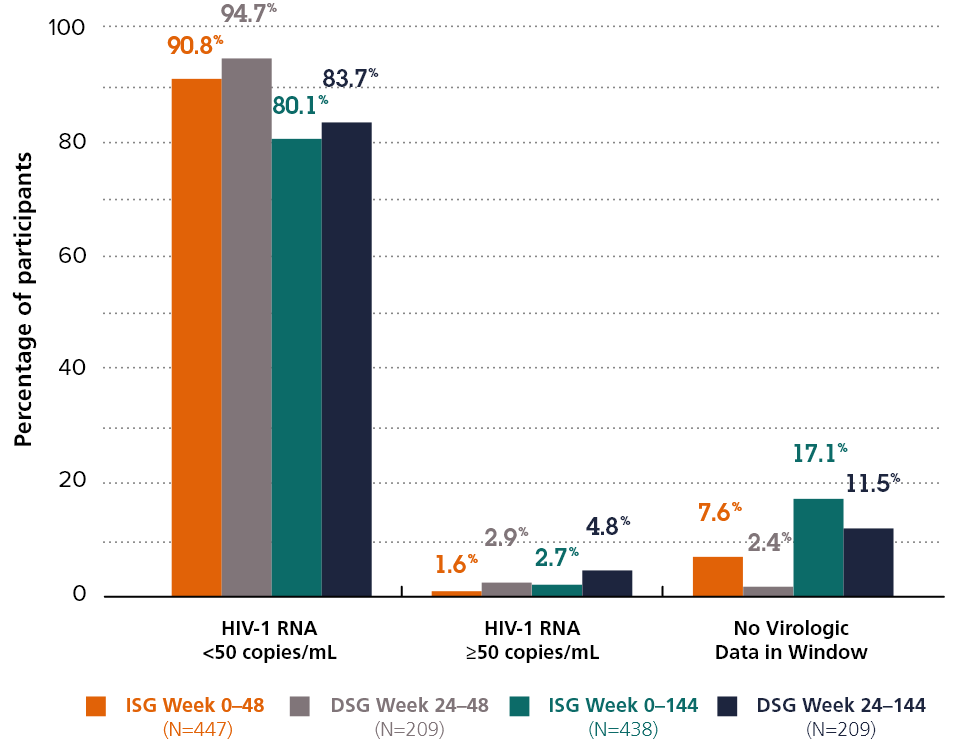

80.1% vs. 83.7%
The proportion of participants with HIV-1 RNA <50 copies/ml at Week 144 in the immediate switch group compared to Week 144 in the delayed switch group.
Adapted from Kumar P. et al. 2021*2
*Participants who completed the base study (Week 48) but did not continue to extension-1 (up to Week 144) were excluded from the efficacy analysis after week 48.

DRIVE-SHIFT was a randomised, open-label, non-inferiority trial evaluating the efficacy and safety of virologically suppressed adults who switched from a baseline regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) in combination with a protease inhibitor (PI) plus either ritonavir or cobicistat, or elvitegravir plus cobicistat, or an NNRTI to DELSTRIGO. Subjects must have been virologically suppressed (HIV-1 RNA <50 copies/mL) on their baseline regimen for at least 6 months prior to trial entry, with no history of virologic failure. Subjects were randomised to either switch to DELSTRIGO at baseline (n=447, immediate switch group [ISG]), or stay on their baseline regimen until Week 24, at which point they switched to DELSTRIGO (n=223, delayed switch group [DSG]).
Efficacy of doravirine in treatment-naïve adults with HIV-1: 4 years of experience from the DRIVE-FORWARD and DRIVE-AHEAD clinical trials3
Efficacy of DOR have been demonstrated through Week 96 in two Phase 3 studies in antiretroviral-naïve adults with HIV-14,5

DRIVE-FORWARD:4 DOR versus ritonavir-boosted darunavir (DRV/r), both with 2 nucleos(t)ide reverse transcriptase inhibitors (NRTIs)4

DRIVE-AHEAD:5 DOR/3TC/TDF (fixed-dose tablet) versus efavirenz/emtricitabine/TDF (EFV/FTC/TDF; fixed-dose tablet)5
All participants who completed Week 96 had the option to continue DOR treatment in a 96-week open-label extension.3
DOR/3TC/TDF = DELSTRIGO (doravirine/lamivudine/tenofovir disoproxil fumarate).
Efficacy outcomes through Week 192: DOR regimen3
Participants with HIV-1 RNA <50 copies/mL combined studies
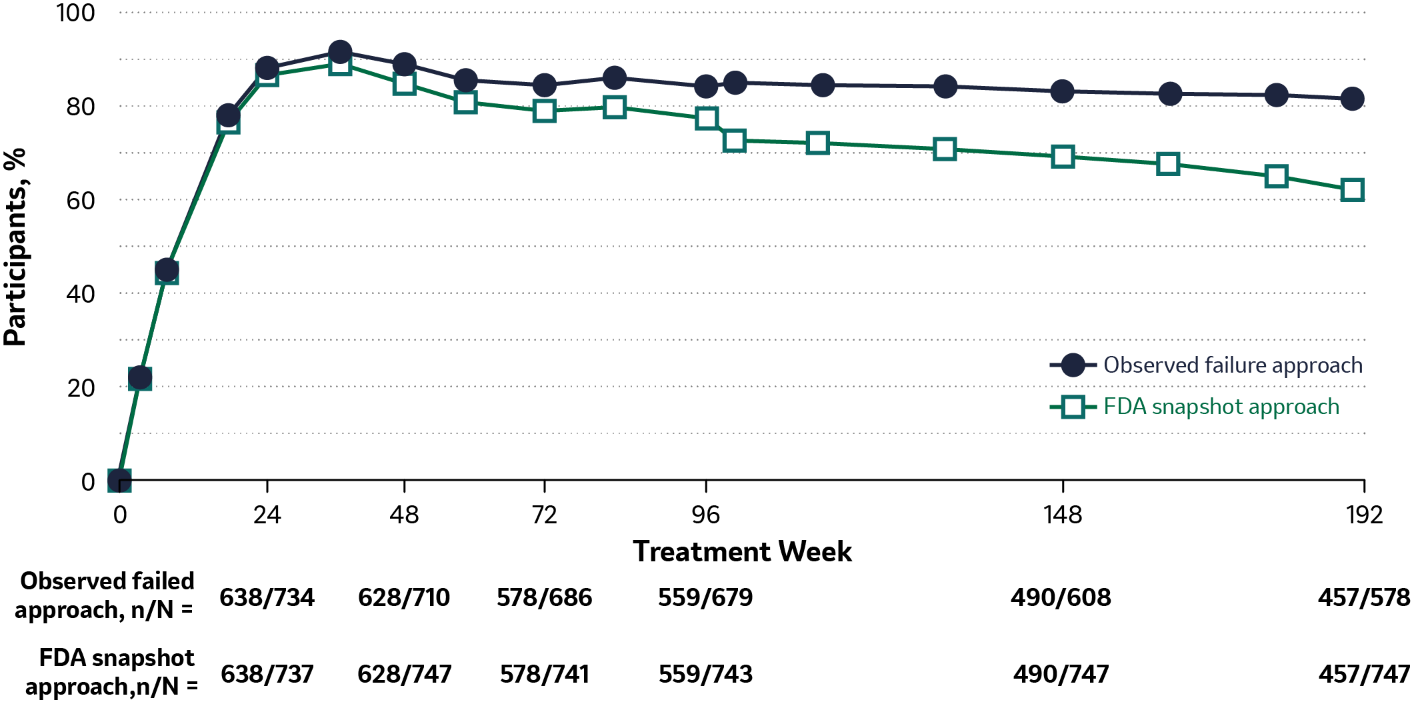
79.1% vs. 61.2%
The proportion of participants with HIV-1 RNA <50 copies/ml at Week 192 in the observed failure approach versus the FDA snapshot approach.
Adapted from Molina J M et al, 20213
- At Week 192, HIV-1 RNA <50 copies/mL was maintained in most participants: 79.1% by observed failure approach and 61.2% by FDA snapshot approach
- At Week 192, the mean increase from baseline in CD4+ T-cell count was 236.2 cells/mm3 (95% CI: 216.8, 255.6)
Observed failure approach: baseline value was carried forward for virologic failures; other missing values were excluded from analysis; NRTIs were TDF/FTC or abacavir/3TC.

Abbreviations
3TC = Lamivudine, ABC = Abacavir, DOR=Doravirine, DRV/r = ritonavir-boosted Darunavir, EFV=Efavirenz, FTC=Emtricitabine, NRTI=Nucleoside Reverse Transcriptase Inhibitor, NNRTI – Non-Nucleoside Reverse Transcriptase Inhibitor, TDF=tenofovir Disoproxil Fumurate.
References
- Johnson M, Kumar P, Molina JM, et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/ TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr. 2019;81(4):463-472.
- Kumar P et al. Switching to Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (DOR/3TC/TDF) Maintains HIV-1 Virologic Suppression Through Week 144 in the DRIVE-SHIFT Trial. JAIDS Journal of Acquired Immune Deficiency Syndromes. Publish Ahead of Print DOI: 10.1097/QAI.0000000000002642.
- Molina J M, Orkin C et al. Safety and efficacy of doravirine in treatment-naïve adults with HIV-1: 4 years of experience from the DRIVE‑FORWARD and DRIVE‑AHEAD clinical trials. Presented at 2021 European AIDS Conference (EACS), Virtual and London, UK, 27‑30 October 2021.
- Orkin C, Squires K E et al. Doravirine/lamivudine/tenofovir disoproxil fumarate (TDF) versus efavirenz/emtricitabine/TDF in treatment naïve adults with human immunodeficiency virus type 1 infection: week 96 results of the randomized, double blind, phase 3 DRIVE‑AHEAD non inferiority trial. Clin Infect Dis 2020:1–10.
- Molina J M, Squires K E et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naïve adults with HIV-1 (DRIVE‑FORWARD): 96 week results of a randomised, double blind, non-inferiority, phase 3 trial. Lancet HIV 2020;7:16–26.
Supporting documentation
DELSTRIGO® Prescribing Information (United Kingdom) [External link]
PIFELTRO® Prescribing Information (United Kingdom) [External link]
By clicking the links above you will leave the MSD Connect website and be taken to the emc website
