Eligible patients for the NHS Pneumococcal Immunisation Programme
Eligible patients for the NHS Pneumococcal Immunisation Programme
PNEUMOVAX® 23 (pneumococcal polysaccharide vaccine)
Prescribing Information [External link]
Pneumococcal disease poses a burden to the community all year round – not only in winter, unlike seasonal influenza.1
Certain patients are at increased risk of complications
Both at-risk individuals and over-65-year-olds have a higher risk of developing pneumococcal disease, longer duration of hospitalisation and/or higher rates of mortality.2-6
Order and start vaccinating today
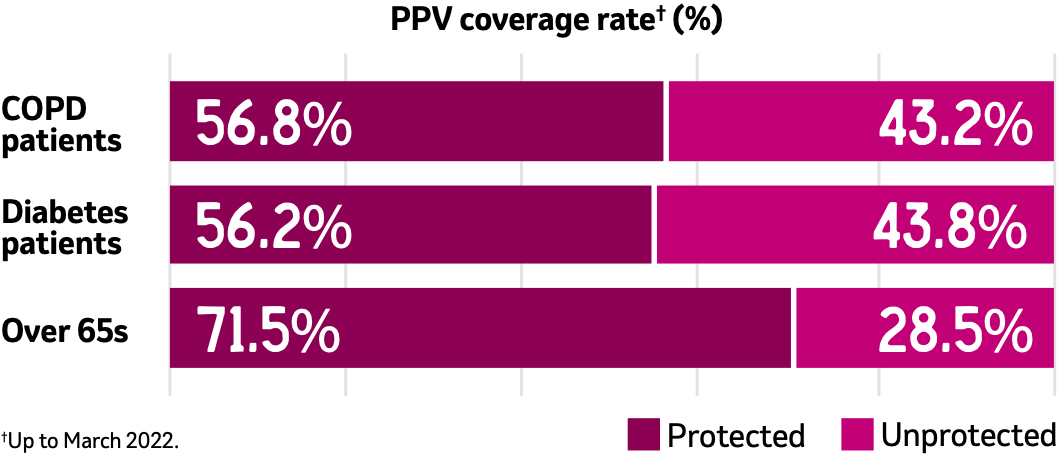
Your patients may be unprotected against pneumococcal disease8
Other clinical risk groups eligible for PPV237
- Asplenia or dysfunction of the spleen
- Diabetes requiring insulin or anti-diabetic medication
- Chronic respiratory disease (chronic respiratory disease refers to chronic lower respiratory tract disease) including COPD
- Immunosuppression
- Chronic heart disease
- Individuals with cochlear implants
- Chronic kidney disease
- Individuals with cerebrospinal fluid leaks
- Chronic liver disease
- Occupational risk
Please refer to the Green Book – Immunisation against infectious disease (Chapter 25)1, for a full list of children aged 2 years and over and adults, considered at risk.7
PPV23 can be administered at your clinic all year round
Order and start vaccinating today7,9
*PNEUMOVAX® 23 (pneumococcal polysaccharide vaccine) is recommended for active immunisation against pneumococcal disease in children aged from 2 years, adolescents and adults.9
COPD = chronic obstructive pulmonary disease; PPV = pneumococcal polysaccharide vaccine.
References
- Torres A, et al. Thorax 2015;70(10):984–989.
- Martins M, et al. BMJ Open Diabetes Res Care. 2016;4:e000181.
- Falcone M, et al. Medicine 2016;95(5):e2531.
- Restrepo MI, et al. Tuberc Respir Dis. 2018;81:187–197.
- Dai RX, et al. BMC Pulm Med. 2018;18(1):12.
- Grant LR, et al. Expert Rev Vaccines. 2021;20(6):691–705.
- The Green Book – Immunisation against infectious disease (January 2020). Chapter 25 – Pneumococcal. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/857267/GB_Chapter_25_pneumococcal_January_2020.pdf.
- UK Health Security Agency. Pneumococcal Polysaccharide Vaccine (PPV) coverage report, England, April 2021 to March 2022. Available at: https://www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-vaccine-coverage-estimates/pneumococcal-polysaccharide-vaccine-ppv-coverage-report-england-april-2021-to-march-2022.
- Pneumovax-23 Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/9692/smpc.
By clicking the links above you will leave the MSD Connect website and be taken to third-party websites
Supporting documentation
PNEUMOVAX® 23 (pneumococcal polysaccharide vaccine)
Prescribing Information
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website



