Over 65s
Over 65s
PNEUMOVAX® 23 (pneumococcal polysaccharide vaccine)
Prescribing Information [External link]


PPV coverage rate in adults aged ≥65 up to March 20223
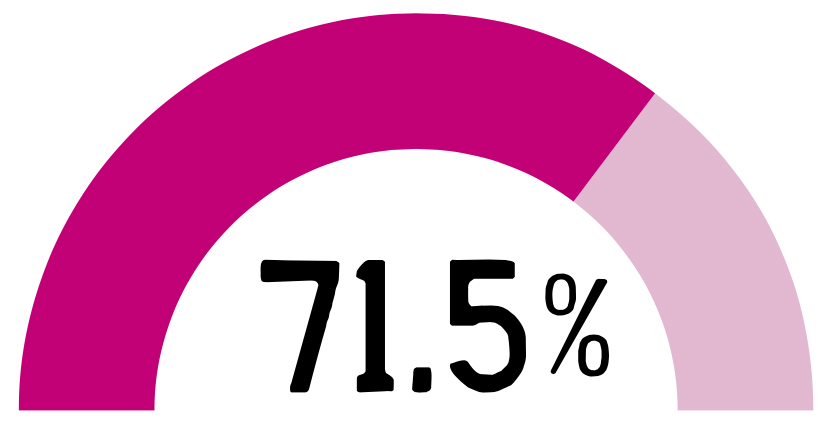

of adults ≥65 years old were unprotected against pneumococcal disease in 20223
Order and start vaccinating today
PNEUMOVAX® 23 (PPV23) can be administered to all patients ≥65 years old at your clinic1,2 – all year round.
Ageing is associated with increased risk and severity of pneumococcal disease4
Over-65-year-olds have a higher risk of developing pneumococcal disease, longer duration of hospitalisation and/or higher rates of mortality.4–8

The increased risk may be due to:4
- A weakened immune system (immunosenescence)
- Presence of comorbidities/other health conditions

Pneumonia is one of the leading causes of morbidity and mortality from infection in elderly patients9

Of the ~187,000 hospital admissions for community-acquired pneumonia (CAP) in 2019, >70% of patients were over 65 years of age10
You can help protect your patients from pneumococcal disease all year round*1

PNEUMOVAX® 23 offers immunisation against pneumococcal infections in all adults over 65 years*1
You only need a single 0.5 mL dose (injected subcutaneously or intramuscularly) for most of your eligible patients.‡1,2
Efficacy data
Based on real-world evidence:
- Most healthy adults develop a good antibody response to a single dose of PPV23 by the third week following immunisation1
- PPV23 is shown to be moderately effective at reducing CAP among UK patients aged ≥65, in the two years after vaccination11
- Vaccine effectiveness is shown to be maintained, and may increase, in the oldest age groups in step with increasing susceptibility to CAP11
- A meta-analysis revealed significant VE of PPV23 against both IPD and pneumococcal pneumonia by any serotype in the elderly, comparable to the efficacy of PCV13 against vaccine-serotype disease in a recent clinical trial in elderly people‡12
- The efficacy of PPV23 was established for pneumococcal pneumonia in RCTs that were conducted among novice gold miners in South Africa2
- The results from one epidemiologic study suggest that vaccination may provide protection for at least 9 years after receipt of the initial dose of vaccine2
For more information on the safety of PNEUMOVAX® 23, visit our Safety information page
PPV23 is in stock§ and available to order via ImmForm as part of the NHS National Immunisation Programme, and can be given to your COPD patients at any time during the year2,13,14
*PNEUMOVAX® 23 (pneumococcal polysaccharide vaccine) is recommended for active immunisation against pneumococcal disease in children aged from 2 years, adolescents and adults2
†Revaccination may be needed in certain at-risk groups. The timing and need of revaccination should be determined on the basis of official recommendations and in line with the SmPC1,2
‡A systematic review and meta-analyses of RCTs and observational studies investigating the efficacy of PPV23 against the specific outcomes PP and IPD in people aged ≥60 years living in industrialized countries. Overall, 4 clinical trials
(3 RCTs and one pseudo-randomized trial) and 13 observational studies were included12
§Subject to supply
CAP = community acquired pneumonia; IPD = invasive pneumococcal disease; PCV = pneumococcal conjugate vaccine; PP = pneumococcal pneumonia; PPV = pneumococcal polysaccharide vaccine; RCT = randomised controlled trial; SmPC = summary of product characteristics; VE = vaccine efficacy
References
- The Green Book – Immunisation against infectious disease (January 2020). Chapter 25 – Pneumococcal. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/857267/GB_Chapter_25_pneumococcal_January_2020.pdf.
- Pneumovax-23 Summary of Product Characteristics.
- UK Health Security Agency. Pneumococcal Polysaccharide Vaccine (PPV) coverage report, England, April 2021 to March 2022. Available at: https://www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-vaccine-coverage-estimates/pneumococcal-polysaccharide-vaccine-ppv-coverage-report-england-april-2021-to-march-2022.
- Grant LR, et al. Expert Rev Vaccines. 2021;20(6):691–705.
- Martins M, et al. BMJ Open Diabetes Res Care. 2016;4:e000181.
- Falcone M, et al. Medicine 2016;95(5):e2531.
- Restrepo MI, et al. Tuberc Respir Dis. 2018;81:187–197.
- Dai RX, et al. BMC Pulm Med. 2018;18(1):12.
- Chebib N, et al. Aging Clin Exp Res. 2021;33(4):1091–1100.
- Campling J, et al. J Med Econ. 2022;25(1):912–918.
- Streeter AJ, et al. PLoS One. 2022;17(10):e0275642.
- Falkenhorst G, et al. PLoS One. 2017;12(1): e0169368.
- UK Health Security Agency. The complete routine immunisation schedule from February 2022.
- National Institute for Health and Care Excellence. BNF Pneumococcal vaccine. Available at: https://bnf.nice.org.uk/treatment-summaries/pneumococcal-vaccine/.
By clicking the links above you will leave the MSD Connect website and be taken to third party websites
Supporting documentation
PNEUMOVAX® 23 (pneumococcal polysaccharide vaccine)
Prescribing Information
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website
