KEYTRUDA monotherapy in HNSCC
KEYTRUDA® (pembrolizumab) in first-line head and neck squamous cell carcinoma (HNSCC)
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic or unresectable recurrent (M/uR) HNSCC in adults whose tumours express PD-L1 with a CPS ≥1.1
With KEYTRUDA monotherapy in the KEYNOTE-048 trial…
Click on each tab below to explore data from the KEYNOTE-048 final analysis and 4-year post-hoc analysis:
KEYNOTE-048 original study design: KEYTRUDA ± chemotherapy vs. EXTREME1,3
Multicentre, randomised, open-label, active-controlled Phase III study
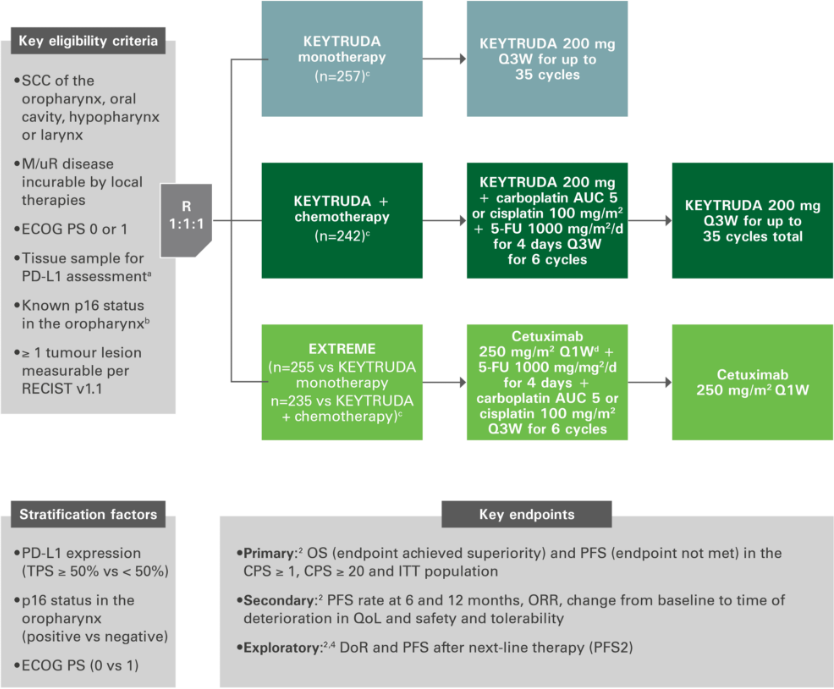
Figure adapted from references 1 to 4. aAssessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent). TPS = % of tumour cells with membranous PD-L1 expression. bAssessed using the CINtec p16 histology assay (Ventana), cutpoint for positivity = 70%. cFollowing a loading dose of 400 mg/m2; after completion of platinum agent and 5-FU, cetuxumab could be continued until PD for patients with stable disease. dCarboplatin AUC 5 OR cisplatin 100 mg/m2 + 5-FU 1000 mg/m2/day for 4 days for 6 cycles of 3 weeks.
Overall survival with KEYTRUDA monotherapy at 2 years in the CPS ≥1 population vs. EXTREME2
More than 1 in 4 patients were alive at 2 years.2
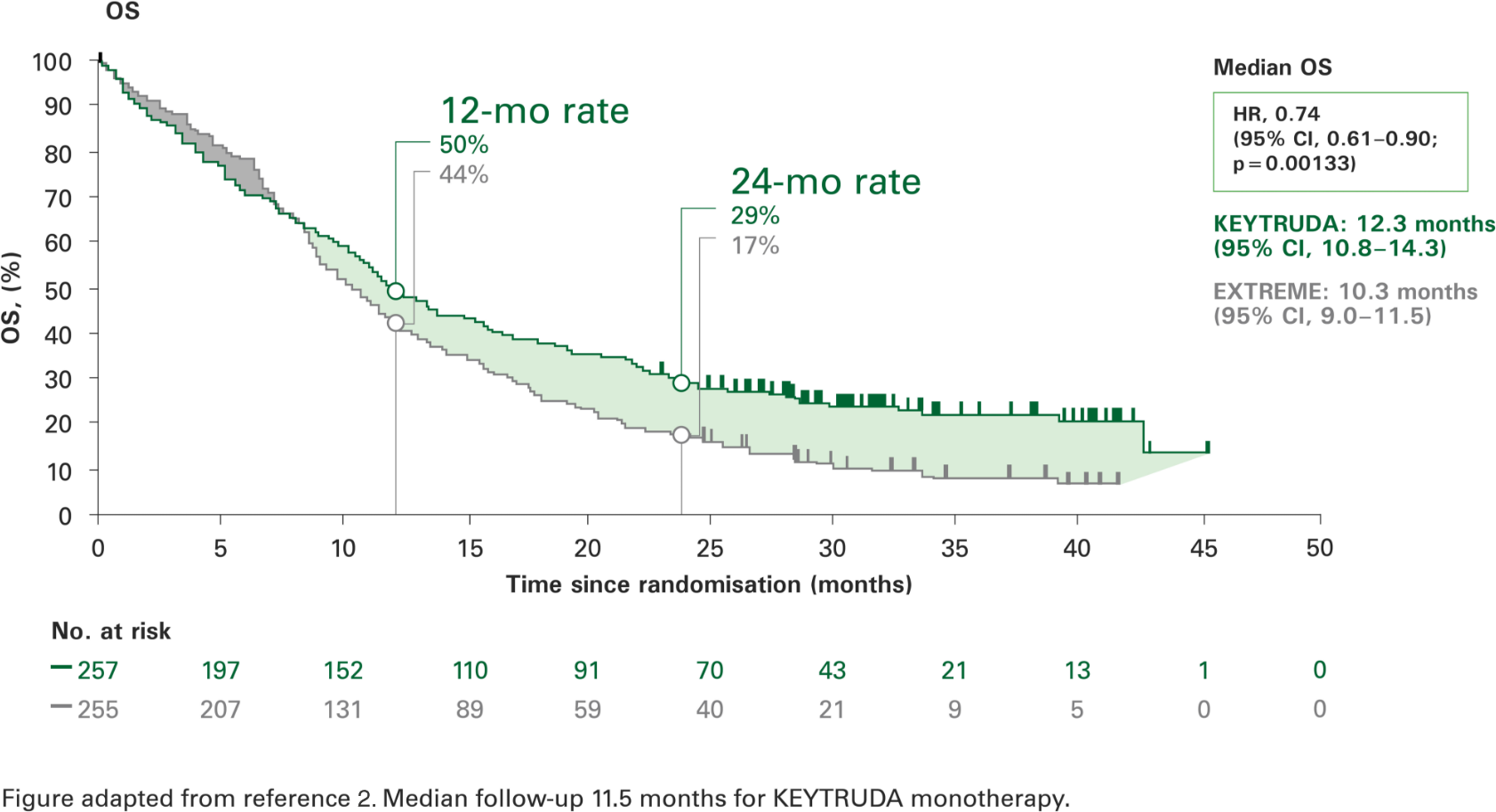
KEYTRUDA monotherapy resulted in a significantly longer overall survival vs. EXTREME and a 26% reduction in the risk of death.2
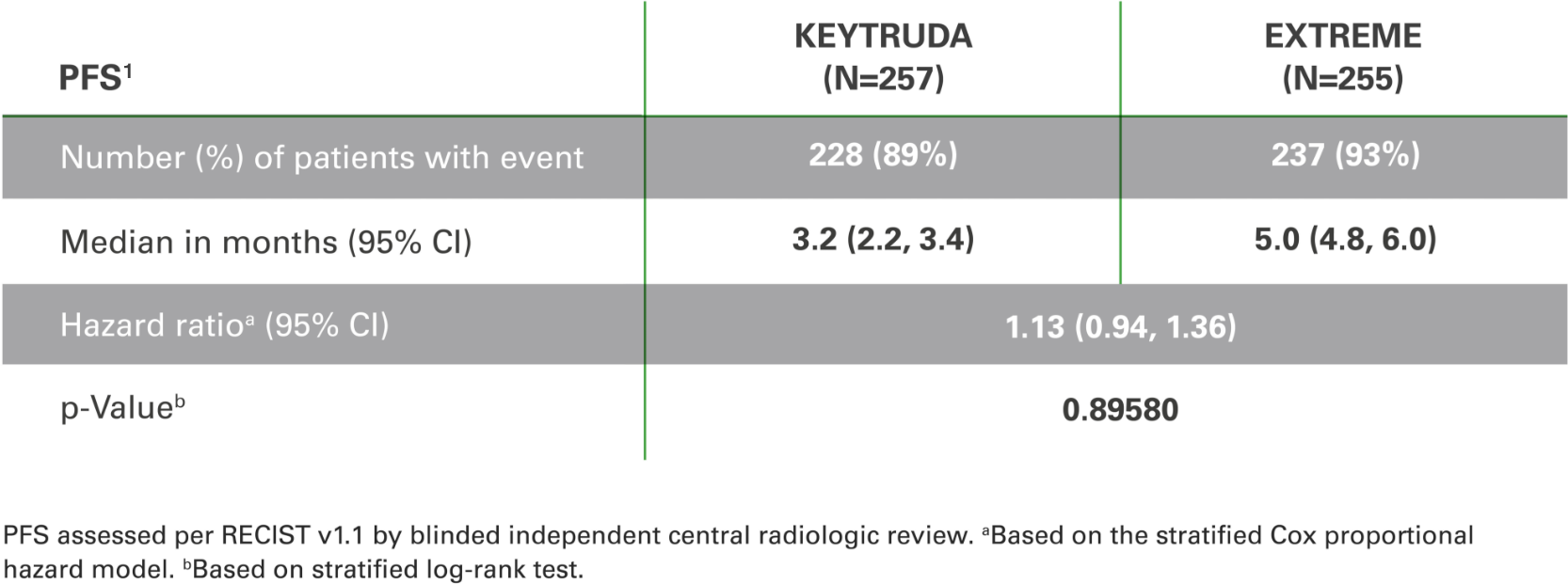
Progression-free survival with KEYTRUDA monotherapy at 2 years in the
CPS ≥1 population vs. EXTREME1,3
PFS was not significantly different vs. EXTREME1,3
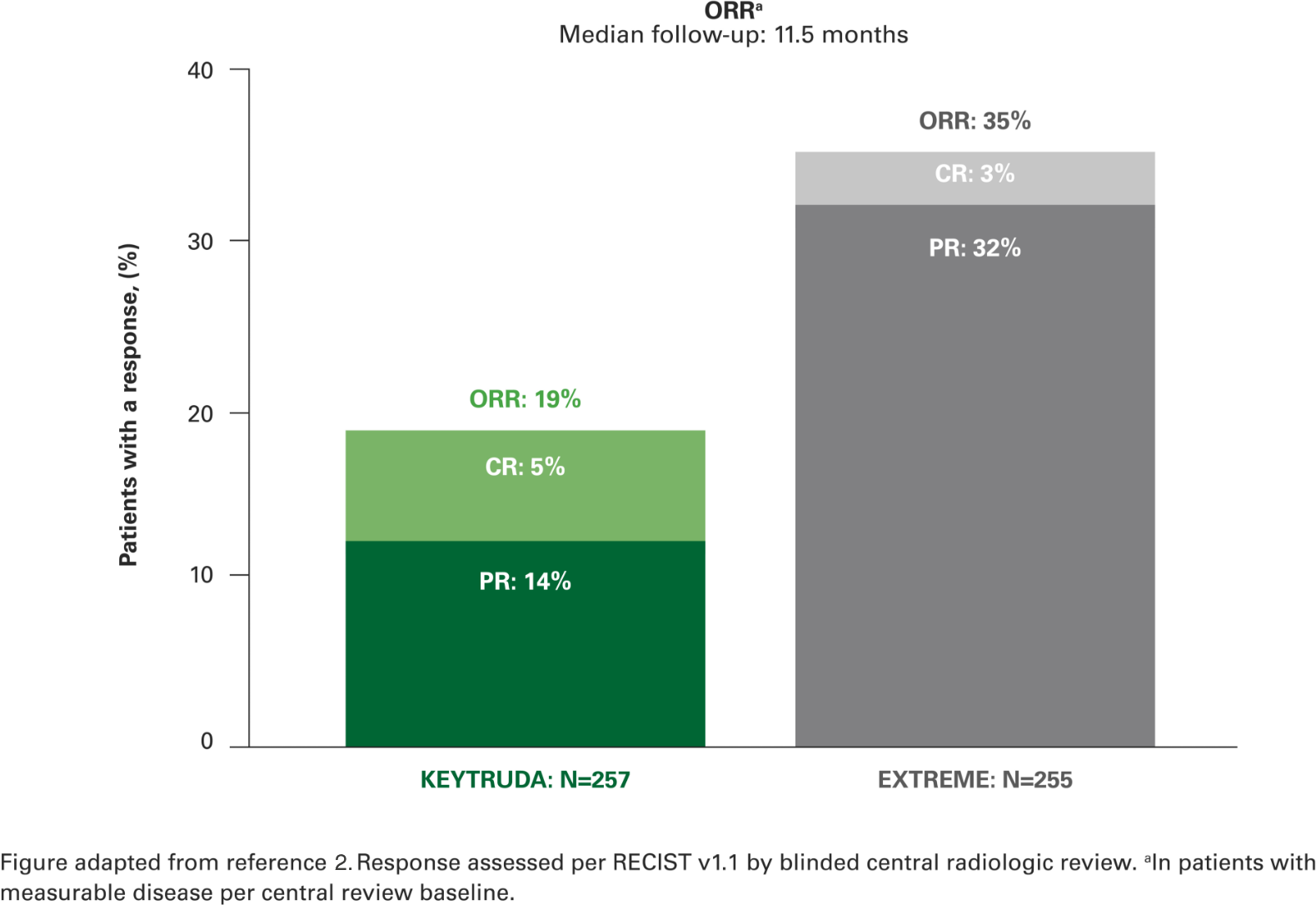
Overall response rate with KEYTRUDA monotherapy at 2 years in the CPS ≥1 population vs. EXTREME2
Almost 1 in 5 patients had a complete or partial response to KEYTRUDA, with a median follow-up of 11.5 months.2
A further 28% of patients in the KEYTRUDA monotherapy arm and 33% of patients in the EXTREME arm had stable disease.2
Duration of response with KEYTRUDA monotherapy at 2 years in the CPS ≥1 population vs. EXTREME2
There was a sustained response with KEYTRUDA monotherapy,
with a median of 2 years.2
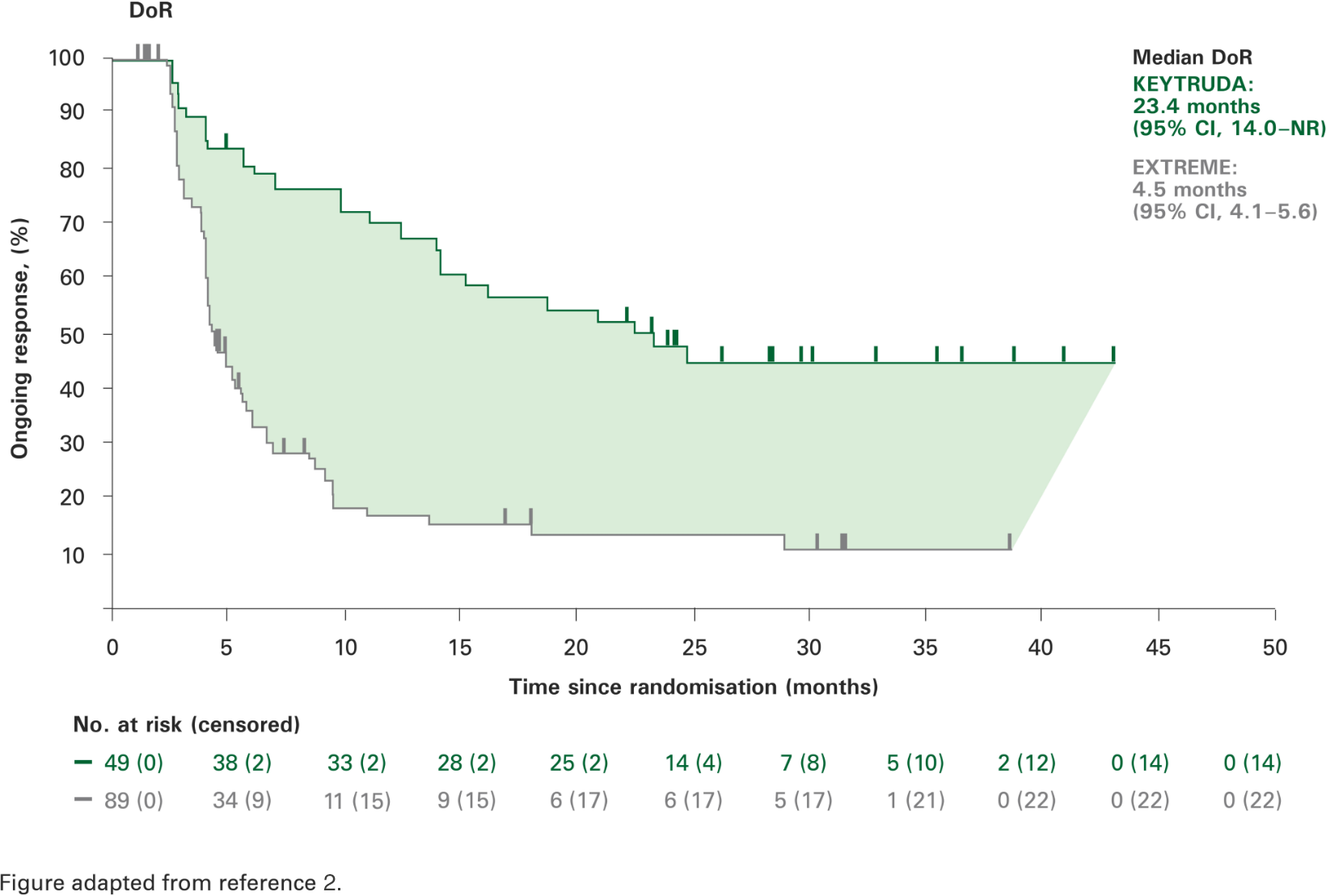
Secondary and exploratory endpoints were not powered for statistical comparison
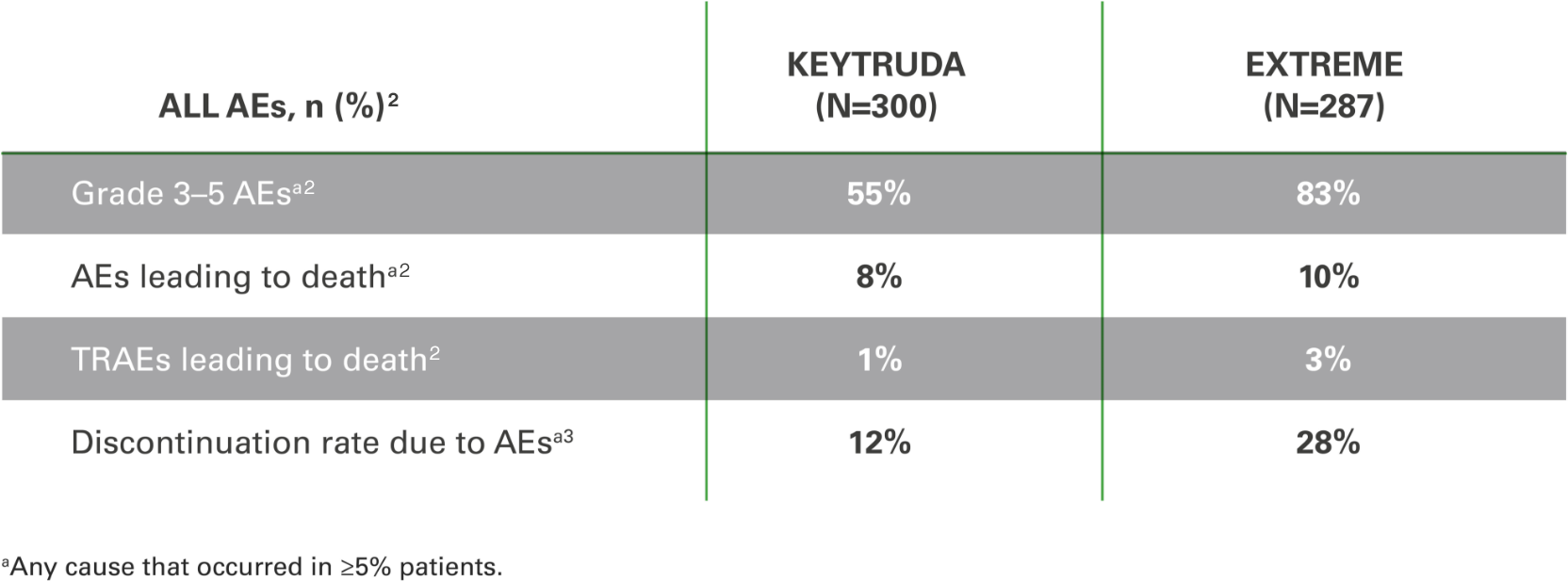
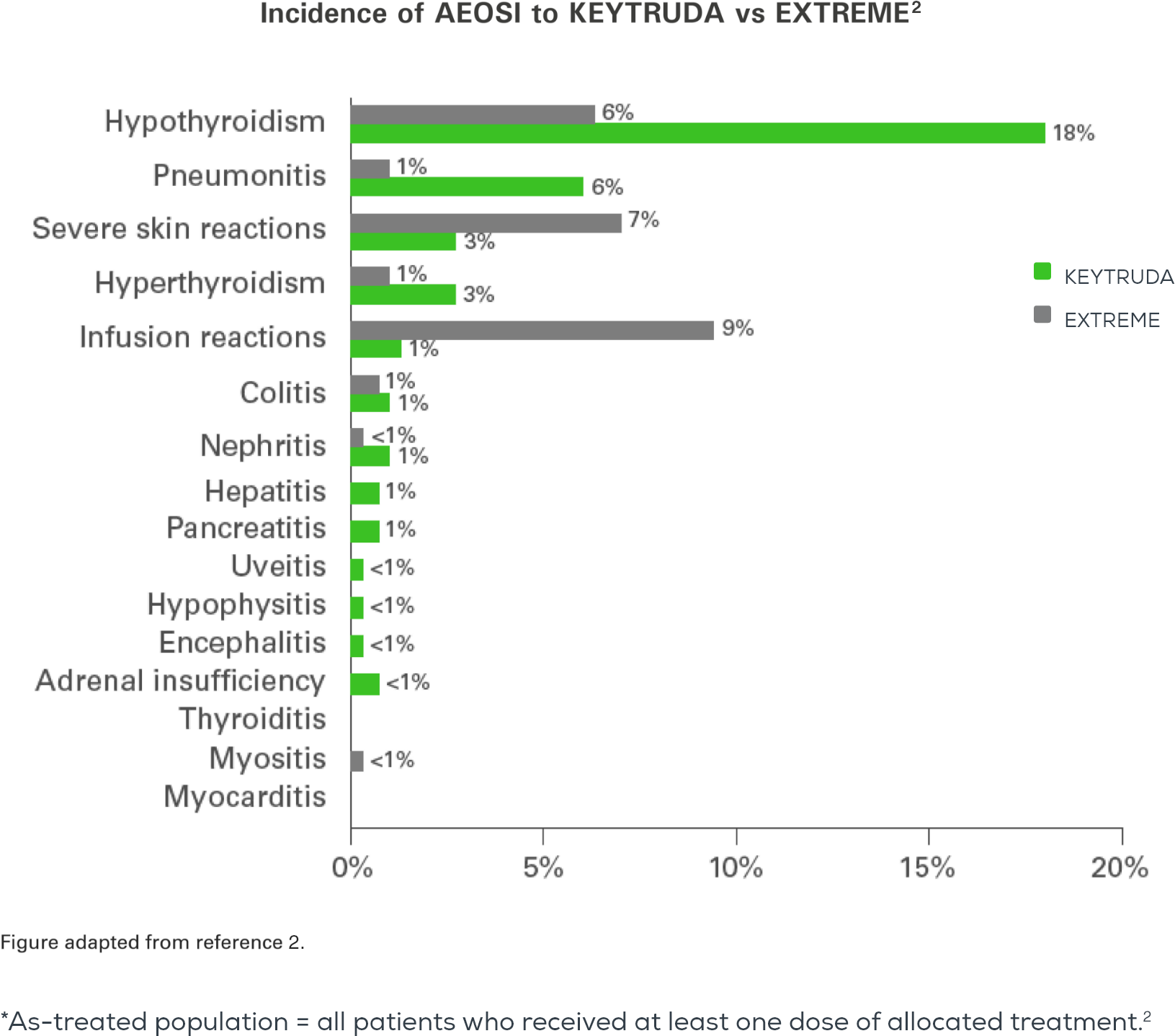
Adverse events with KEYTRUDA monotherapy at 2 years in the as-treated population* vs. EXTREME2
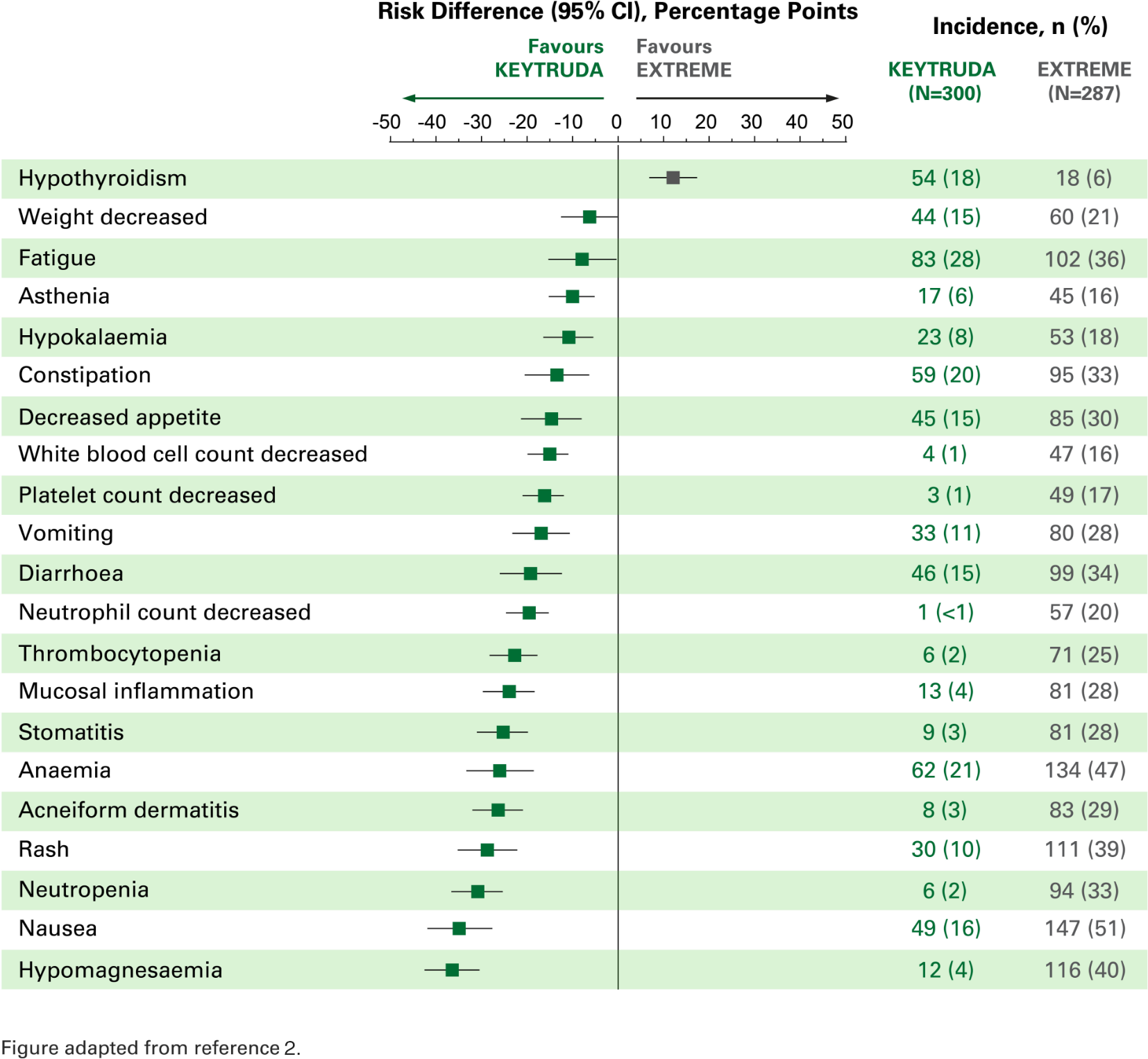
The overall safety profile for KEYTRUDA was favourable vs. EXTREME, with the exception of hypothyroidism and pneumonitis.2
Risk difference for adverse events of any cause with incidence ≥15%2
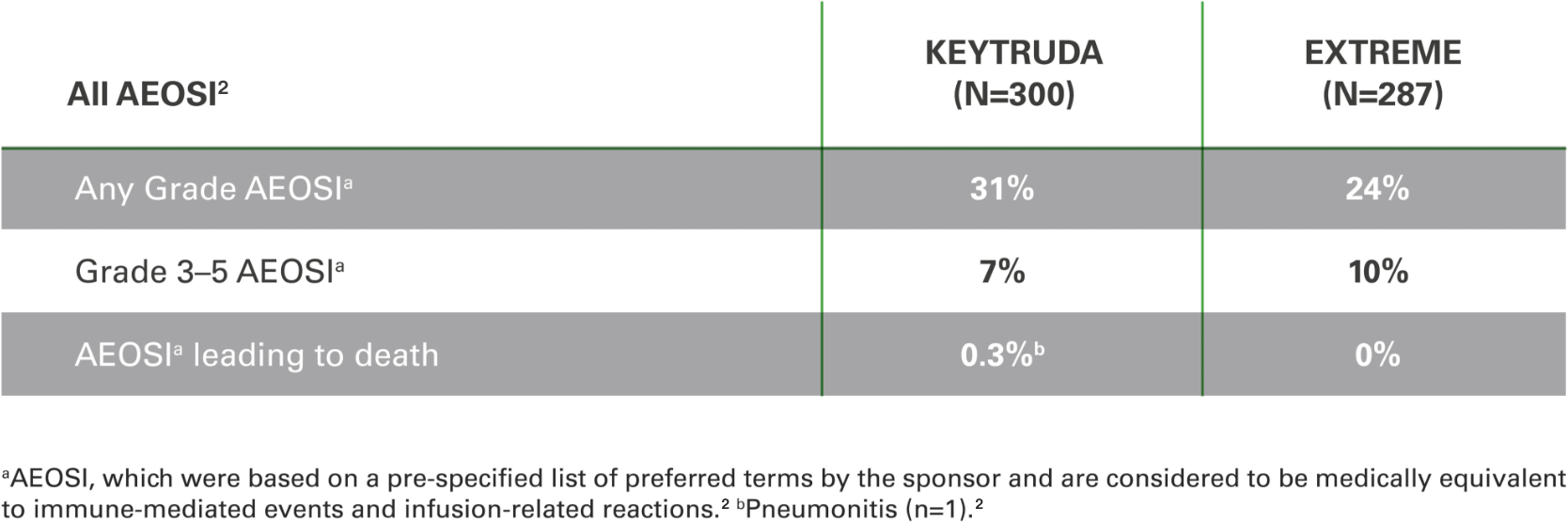
Adverse events of special interest (AEOSI)
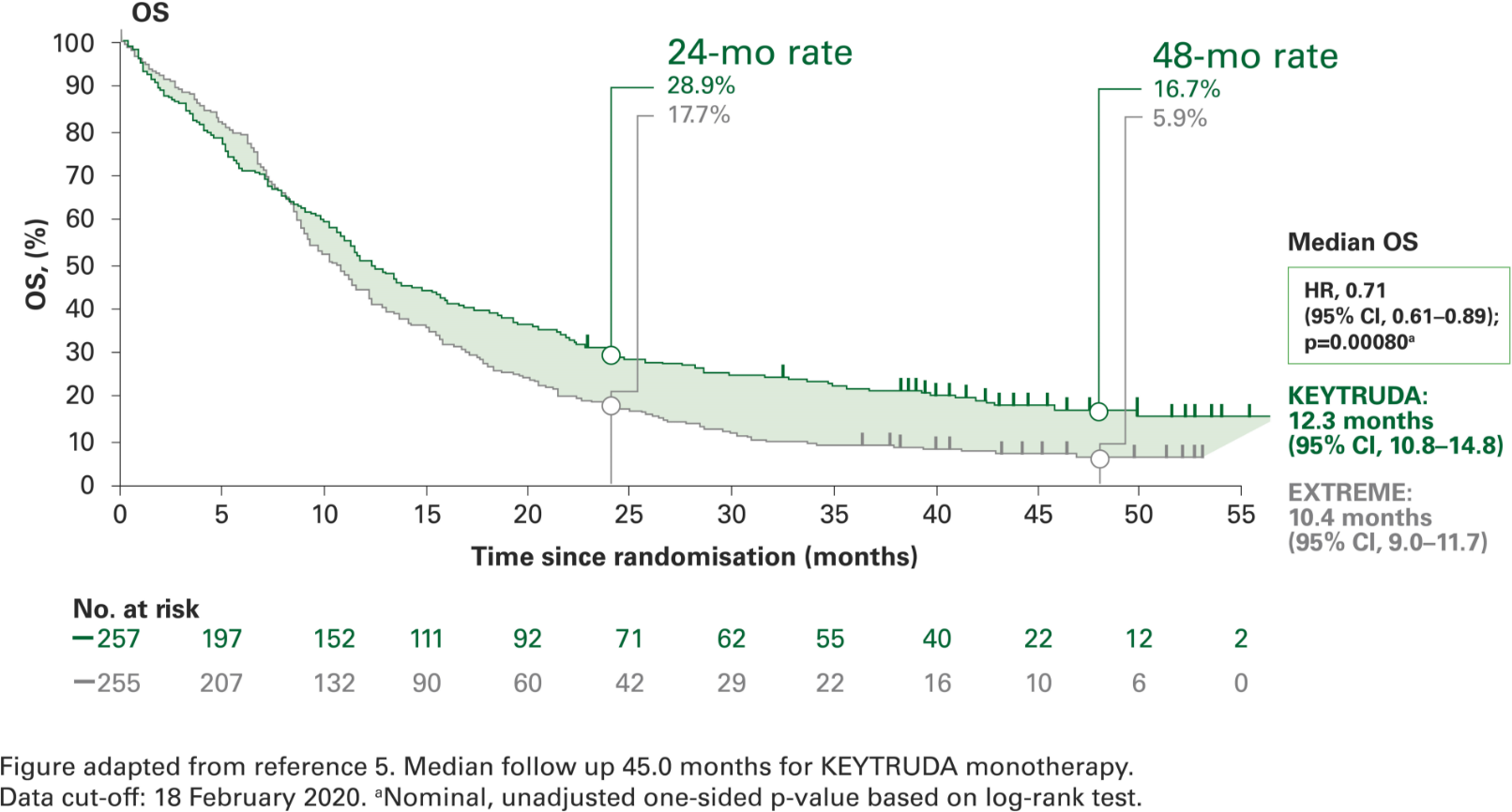
Overall survival with KEYTRUDA monotherapy at 4 years in the CPS ≥1 population vs. EXTREME5
Post-hoc analyses were not powered for statistical comparison
1 in 6 patients were alive at 4 years with KEYTRUDA vs. 1 in 17 with EXTREME5
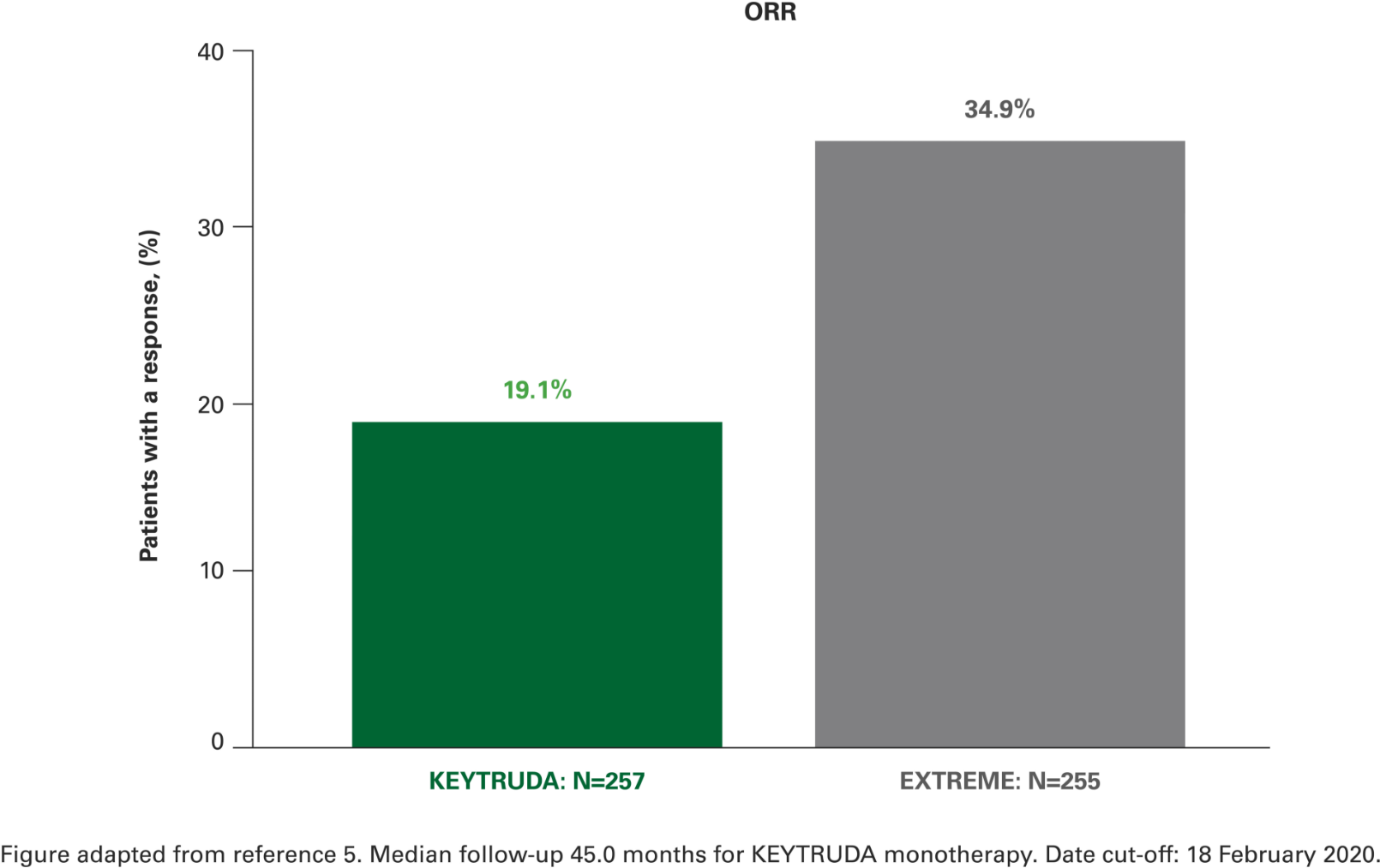
Overall response rate with KEYTRUDA monotherapy at 4 years in the CPS ≥1 population vs. EXTREME5
Post-hoc analyses were not powered for statistical comparison
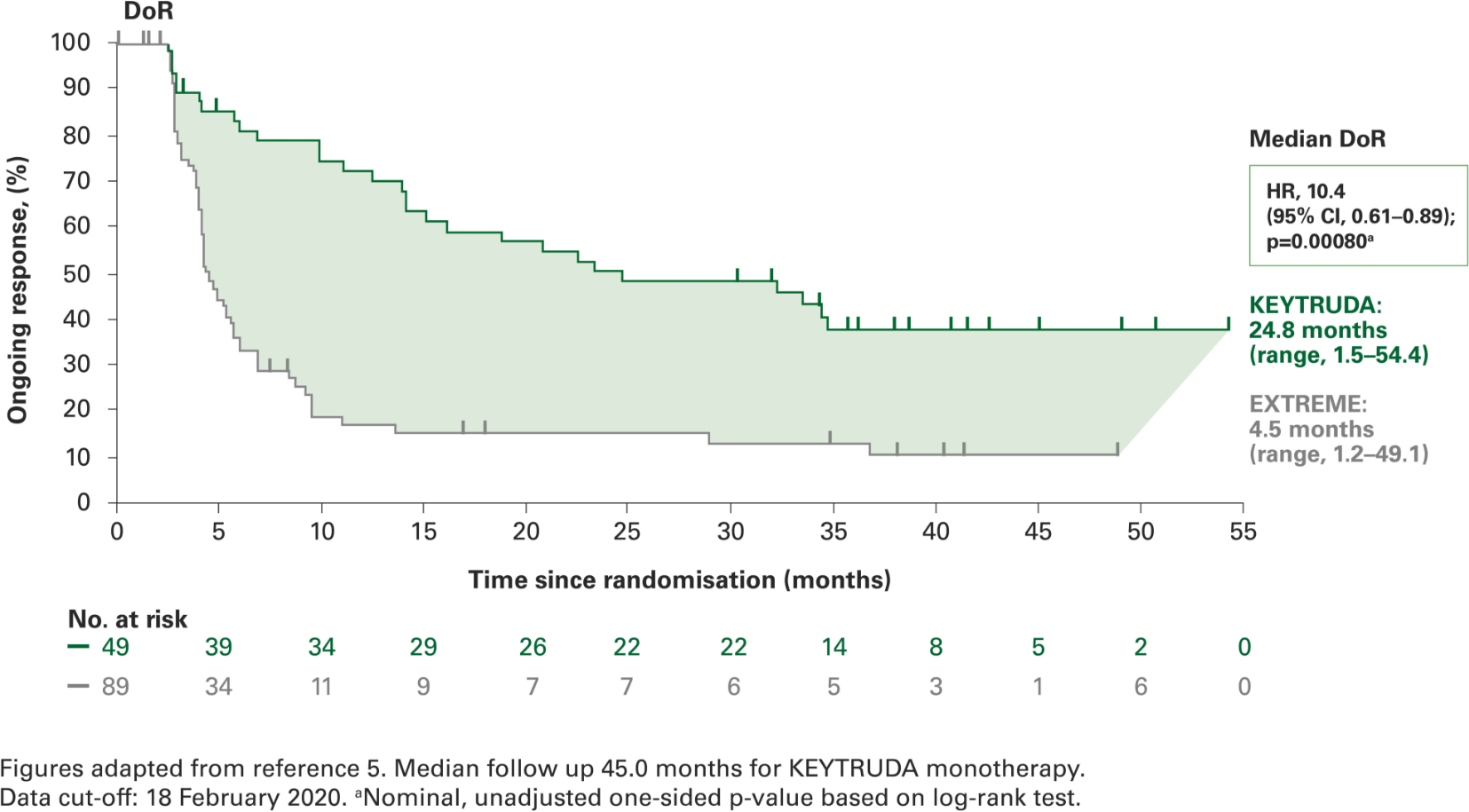
Duration of response with KEYTRUDA monotherapy at 4 years in the CPS ≥1 population vs. EXTREME5
Post-hoc analyses were not powered for statistical comparison
Treatment-related adverse events (TRAEs) with KEYTRUDA monotherapy at 4 years in the as-treated population* vs. EXTREME5
TRAEs remained consistent with known safety profiles5
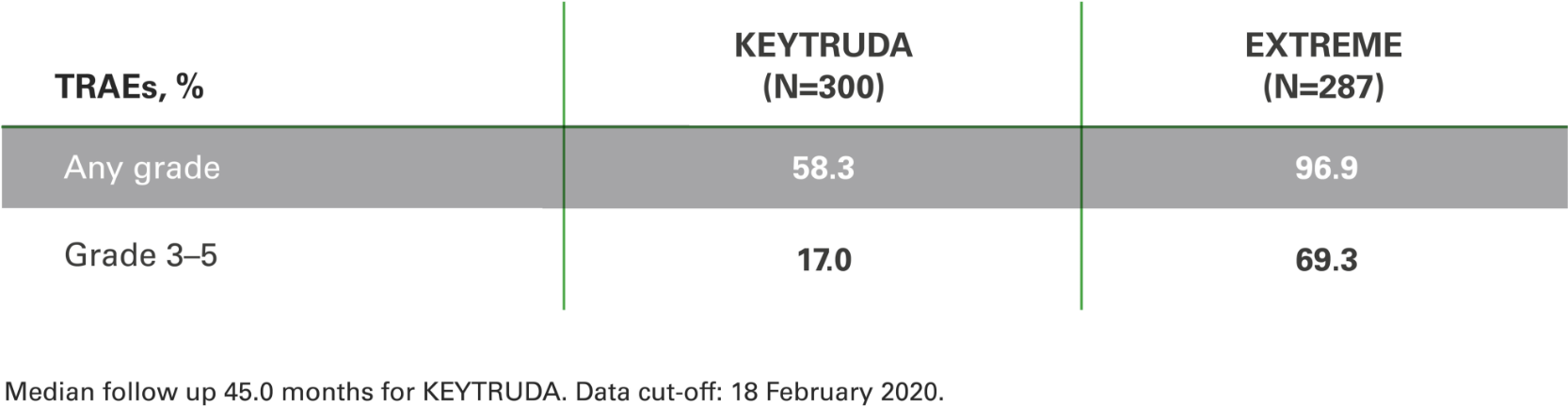
Safety data originates from the total population and could
not be separated by PD-L1 expression levels.
*As-treated population = all patients who received at least one dose of allocated treatment.5
To find out more about the different types of patients who could benefit from KEYTRUDA, arrange a discussion with an MSD representative
Discover all the data for KEYTRUDA monotherapy in M/uR HNSCC

Discover all the data for KEYTRUDA in M/uR HNSCC (KEYNOTE-048 full results slide deck)
Prescribing Information (Great Britain) &
Prescribing Information (Northern Ireland)
[External links]
KEYTRUDA dosing information
With two options available, choose the appropriate dosing regimen for your patients1,3
KEYTRUDA alone or in combination with chemotherapy

OR

Both doses are administered as IV infusions over 30 minutes
The study that led to the approval of the Q6W for monotherapy and combination patients assessed the 400 mg Q6W dosing schedule based on an exposure-response evaluation using modelling and simulation. It concluded that 400 mg Q6W dosing regimen for KEYTRUDA monotherapy and combination is predicted to have a similar efficacy and safety profile as the approved 200 mg Q3W dosing regimen.
PD-L1 testing supports a targeted treatment approach for all appropriate patients with M/uR HNSCC*1
CPS ≥1 in the KEYNOTE-048 study1

*The KEYNOTE-048 study employed the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA, USA)1,6
KEYNOTE-048 trial was a multicentre, randomised, open-label, active-controlled Phase III study.
KEYTRUDA monotherapy; EXTREME n=255.
Abbreviations
5-FU = 5-Fluorouracil; AE = Adverse Event; AEOSI = Adverse Events Of Special Interest; AUC 5 = Desired Carboplatin Exposure of 5 mg/ml; CI = Confidence Interval; CPS = Combined Positive Score; CR = Complete Response; DoR = Duration Of Response; ECOG PS = Eastern Cooperative Oncology Group Performance Status; ESMO = European Society For Medical Oncology; EXTREME = Cetuximab + 5-Fluorouracil + Platinum-Based Chemotherapy; HNSCC = Head And Neck Squamous Cell Carcinoma; HR = Hazard Ratio; IHC = Immunohistochemistry; ITT = Intention To Treat; IV = Intravenous; mo = Month; M/uR = Metastatic Or Unresectable Recurrent; No. = Number; NR = Not Reported; ORR = Objective Response Rate; OS = Overall Survival; P16 = Cyclin-Dependent Kinase Inhibitor 2A; PD = Progressive Disease; PD-1 = Programmed Cell Death-1; PD-L1 = Programmed Death Ligand-1; PFS = Progression-Free Survival; PFS2 = Time From Randomisation To Objective Tumour Progression On Next-Line Treatment Or Death From Any Cause; PR = Partial Response; Q1W = Every Week; Q3W = Every 3 Weeks; Q6W = Every 6 Weeks; QoL = Quality Of Life; R = Randomised; RECIST = Response Evaluation Criteria In Solid Tumours; SCC = Squamous Cell Carcinoma; TPS = Tumour Proportion Score; TRAE = Treatment-Related Adverse Events.
References
- KEYTRUDA Summary of Product Characteristics.
- Burtness B et al. Lancet. 2019:394;1915–28 (suppl. appx.).
- Burtness B et al. Lancet. 2019:394;1915–28.
- Harrington K et al. Presented at American Society of Clinical Oncology (ASCO) Annual Meeting 2020. 29 May–2 June 2020.
- Greil R et al. Presented at ESMO Virtual Congress 2020; 19–21 September 2020.
- Agilent. PD-L1 IHC 22C3 pharmDx Interpretation Manual – HNSCC.
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website.


