Register with MSD Connect
✔ Sign up for events
✔ Complete learning modules
✔ Receive cancer resources
✔ Access additional content
Promotional article for UK Healthcare Professionals only, sponsored by and written on behalf of MSD by
Dr Christina Wilson.
The opinions expressed in this article are those of the author.
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]

Written on 11 January 2022
Head and neck squamous cell cancer (HNSCC) includes cancers from the oral cavity, oropharynx, larynx and hypopharynx subsites. Patients can recur or present with metastatic disease or, as often the case, relapse or progress locoregionally following radical treatment.
Over the last decade, immune checkpoint inhibitor research has progressed in HNSCC,1 with this treatment class being available for use in eligible patient populations.2-4 Robust and efficient diagnostic pathways within hospitals can provide supporting information to allow clinicians to decide appropriate treatment plans.
For KEYTRUDA® (pembrolizumab) usage in HNSCC, we currently have clinical data from the KEYNOTE-048 trial. The KEYNOTE-048 trial investigated pembrolizumab (200 mg every 3 weeks) as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy versus a standard treatment (EXTREME – cetuximab 400 mg/m2 load then 250 mg/m2 once weekly, carboplatin AUC 5 mg/mL/min every 3 weeks or cisplatin 100 mg/m2 every 3 weeks, and 5-FU 1,000 mg/m2/d 4 days continuous every 3 weeks, with a maximum of 6 cycles of platinum and 5-FU) in 754 HNSCC patients with tumours which expressed CPS ≥1 and were naïve to treatment in the recurrent or metastatic setting. Overall survival (OS) and progression free survival (PFS) were the two primary endpoints of the trial. Data demonstrated a statistically significant improvement in OS endpoints, but not in PFS. Pembrolizumab as monotherapy showed a superior median OS of 12.3 months (95% CI 10.8, 14.3) compared with 10.3 months (9.0, 11.5) for standard treatment (12.3 vs. 10.3, HR (95% CI) 0.74 [0.61, 0.90]). Pembrolizumab in combination therapy demonstrated a superior median OS of 13.6 months (10.7, 15.5) compared with 10.4 months (9.1, 11.7) for those on standard treatment (13.6 vs. 10.4, HR (95% CI) 0.65 [0.53,0.80]: p=0.0001).1
The most common adverse events with pembrolizumab alone were fatigue and anaemia; the most common treatment-related adverse events were fatigue and hypothyroidism. Anaemia and nausea were the most common adverse events of any cause and those attributed to study treatment with pembrolizumab with chemotherapy and cetuximab with chemotherapy. Pembrolizumab alone was associated with a greater risk of hypothyroidism than was cetuximab with chemotherapy, whereas cetuximab with chemotherapy was associated with a greater risk of 20 adverse events. Pembrolizumab with chemotherapy was associated with a greater risk of anaemia, hypothyroidism, and cough than was cetuximab with chemotherapy, whereas risks of hypokalaemia, hypomagnesaemia, rash, and acneiform dermatitis were greater with cetuximab with chemotherapy.1 You can see the KEYTRUDA SmPC and more safety/efficacy data here for Great Britain, and here Northern Ireland.
For the PD-L1 testing in KEYNOTE-048, the tumour biopsies were analysed and characterised by the combined positive score (CPS). This is defined as the number of PD-L1-positive cells (tumour cells, lymphocytes, and macrophages) divided by the total number of tumour cells × 100. In the trial, a minimum of 100 viable tumour cells had to be present to be a sufficient sample for evaluation. Of the 882 participants, 85% had a PD-L1 CPS of ≥1 and 43% had CPS ≥20.
In November 2020, pembrolizumab (KEYTRUDA) monotherapy was recommended as an option by NICE for untreated metastatic or unresectable recurrent HNSCC, in adults whose tumours express PD-L1 with a CPS ≥1, and is subject to the 2-year clinical stopping rule.2 Just prior to this in September 2020 it was accepted for restricted use by the SMC for first-line in Scotland as monotherapy or in combination with platinum-5-fluorouracil chemotherapy for the same indication.3
As per the licensed indications, PD-L1 biomarker testing with CPS scoring is required for KEYTRUDA usage in HNSCC.5 This enables informed clinical decision-making, both at MDT and outpatient consultations, in order to allow clinicians and patients full information and access into the therapeutic management options. For patients with metastatic disease, it allows discussion of appropriate systemic treatment options to be had at first consultation and avoid potential treatment delays. For patients with locoregional advanced disease it allows the open discussion of all possible treatment options, radical and palliative, with associated response, morbidity and chance of long-term survival to be had with the patient, offering patient-centred care.
To enable eligible patient access to KEYTRUDA in HNSCC, we have worked within the regional multidisciplinary teams and managed clinical networks to commence PD-L1 CPS testing and change our clinical management based on this evidence. Seeing these trials and likely changes were coming through, we highlighted this early to our head and neck pathology colleagues who underwent the lengthy but essential training and validation processes needed to set up our regional PD-L1 CPS head and neck scoring system.
As oncologists, we work closely with our colleagues, particularly those in surgery and pathology, to try to streamline the testing pathway for these patients and avoid delays in management decision and allow optimal consultation with the patient on their full treatment options. This has included:
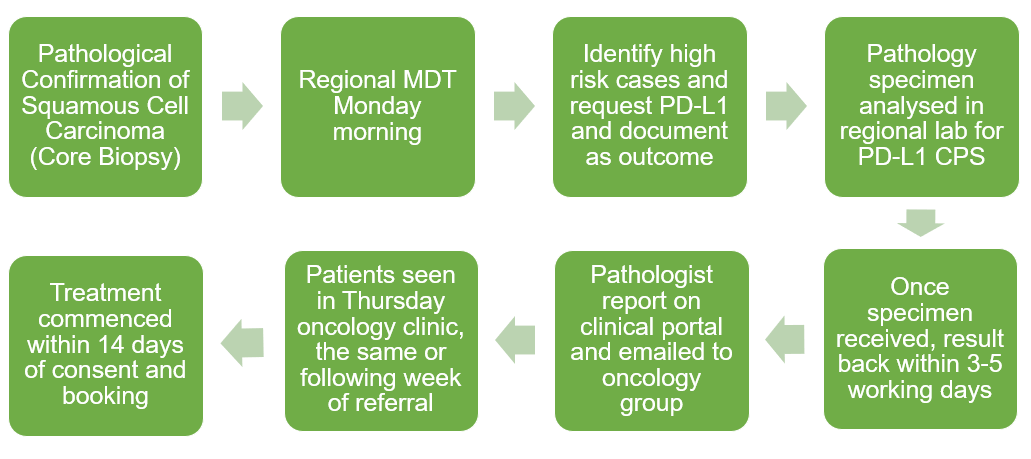
Figure 1: The West of Scotland Regional North H&N MDT Recurrent or Metastatic HNSCC Pathway.
In Scotland, the Scottish Cancer Taskforce National Cancer Quality Steering Group is including a PD-L1 CPS testing target status for appropriate patients as part of their Quality Performance Indicator criteria (QPI15) from this year.6 The aim of this QPI is that a PD-L1 status should be available to inform treatment decisions in patients with head and neck cancer, that are appropriate for treatment, within a time frame from MDT request (specific time frame under consultation).
In conclusion, to provide the best outcomes for patients, we as physicians can have a hand in optimising our PD-L1 head and neck pathways to allow us to have all the diagnostic information to hand in a timely fashion, to make the best clinical management decisions at our MDTs and within the consultations with our patients.
Dr Christina Wilson
Consultant Oncologist
The Beatson West of Scotland Cancer Centre, Glasgow
✔ Sign up for events
✔ Complete learning modules
✔ Receive cancer resources
✔ Access additional content
CPS = Combined Positive Score; H&N = Head And Neck; HNSCC = Head And Neck Squamous Cell Carcinoma; MDT = Multi Disciplinary Team; NICE = The National Institute For Health And Care Excellence; PD-L1 = Programmed Death Ligand-1; QPI = Quality Performance Indicator; SMC = Scottish Medicines Consortium.
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website.
This section of the website contains promotional information intended for UK Healthcare Professionals only. If you are not a UK healthcare professional, please click here.
GB‑NON‑10777 | Date of Preparation: January 2025

This section of MSD Connect is an online portal containing promotional information about MSD pharmaceutical products, therapy area materials and professional resources and is intended for UK Healthcare Professionals.
To contact us please telephone 0208 154 8000 or email medicalinformationuk@msd.com | Privacy Policy | Terms of Use
GB-NON-10696 | Date of Preparation: January 2025
Merck Sharp & Dohme (UK) Limited Registered Office: 120 Moorgate, London, EC2M 6UR, United Kingdom.
Registered in England No. 233687 Copyright © 2025 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Merck Sharp & Dohme (UK) Limited (Tel: 0208 154 8000)

As part of the implementation of the Windsor Framework, from January 1st 2025, all medicines approved by UK authorities will be available across the United Kingdom, including Northern Ireland. Therefore, for medicines on this website that currently have separate prescribing information links for Great Britain (GB) and Northern Ireland (NI), both links will now direct to a single, UK-wide prescribing information for that medicine.
GB-NON-10797 | Date of Preparation: January 2025