About ZEPATIER
About ZEPATIER® (elbasvir and grazoprevir)
Prescribing Information [External link]
ZEPATIER is indicated for the treatment of chronic hepatitis C (CHC) in adult and paediatric patients 12 years of age and older who weigh at least 30 kg1
Please consult the ZEPATIER SmPC before prescribing.
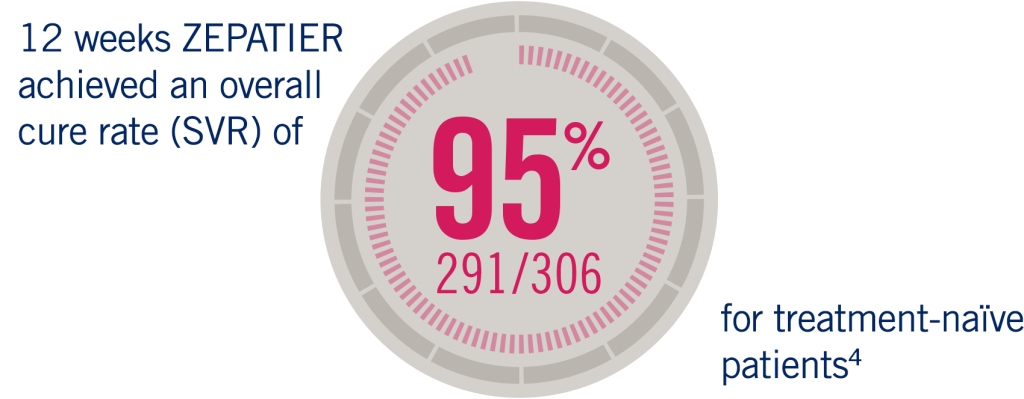
The C-EDGE Treatment Naïve study was an international, randomised, blinded, placebo-controlled, parallel-group trial of a fixed-dose combination of grazoprevir 100 mg/elbasvir 50 mg for treatment-naïve cirrhotic and non-cirrhotic patients with chronic HCV GT1, GT4, or GT6 infections. A historical SVR12 rate was used as the comparator for efficacy.
ZEPATIER has demonstrated efficacy in People who inject drugs2
ZEPATIER for all stages of chronic kidney disease (CKD)3
References
- ZEPATIER Summary of Product Characteristics.
- Dore GL et al, Ann Int Med. 2016;doi:10.7326/M16-0816 [Epub ahead of print].
- Roth D et al. Lancet. 2015:386:1537-1545.
- Zeuzem S et al. Ann Intern Med. 2015; 163:1-13.
Supporting documentation
Prescribing Information [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website

