KEYNOTE-671: KEYTRUDA neoadjuvant therapy plus platinum-based chemotherapy followed by adjuvant monotherapy in early-stage NSCLC (KEYTRUDA perioperative treatment)
Prescribing Information [External link]
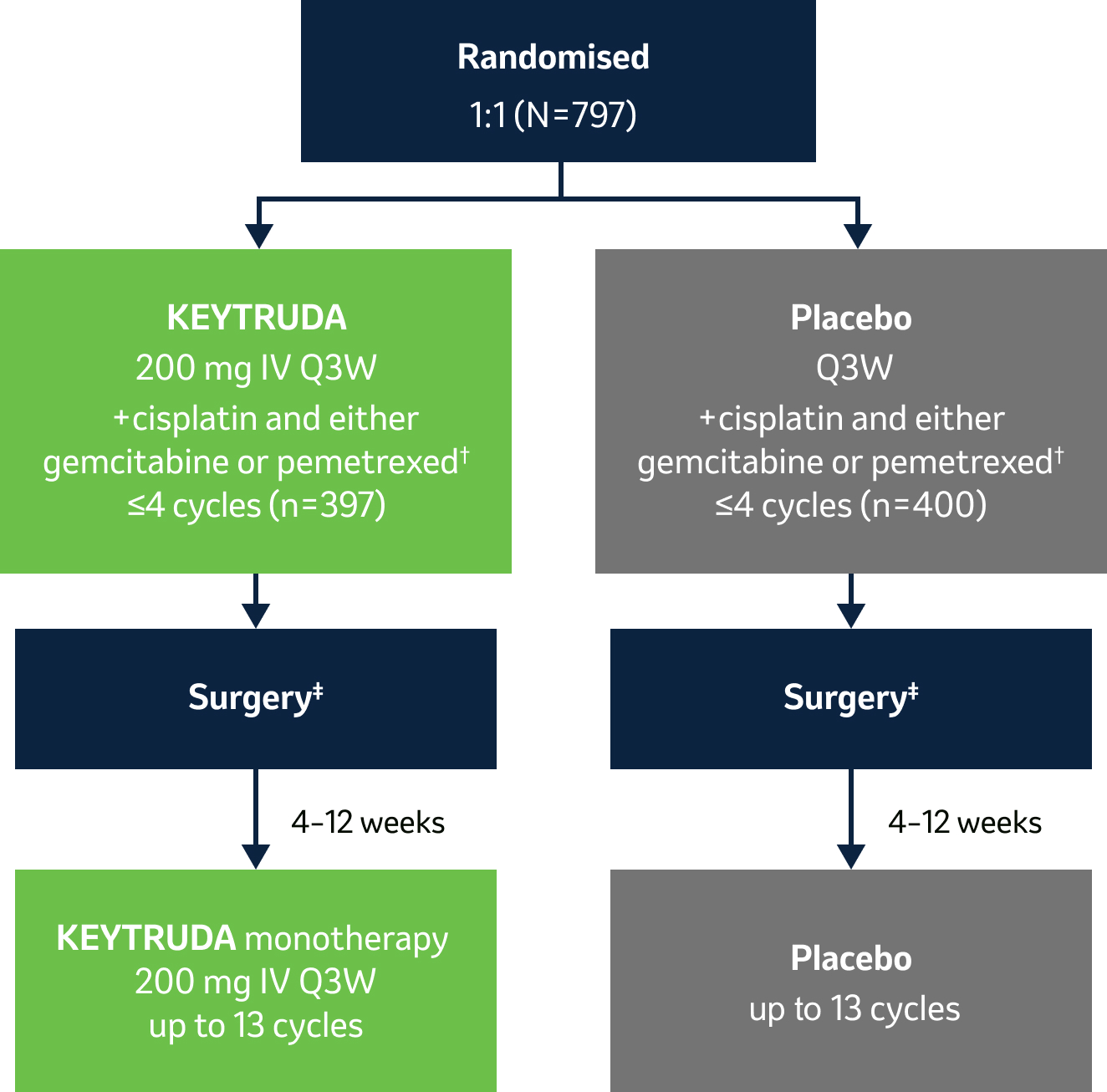
The efficacy and safety of KEYTRUDA were investigated as perioperative treatment in early-stage NSCLC in
KEYNOTE-671
…a Phase III, randomised, double-blind clinical study in 797 patients with resectable Stage II, IIIA or IIIB (N2)* NSCLC.1
During this trial, KEYTRUDA achieved the dual primary endpoints of event-free survival and overall survival – see below for more details.
*Tumour staging system as per AJCC 8th edition.
Licensed indication
KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable non-small cell lung carcinoma at high risk of recurrence in adults.2
Key eligibility criteria:
- Pathologically confirmed, resectable Stage II, IIIA or IIIB (N2) NSCLC as per AJCC 8th edition
- ECOG PS 0–1
- Able to undergo surgery
- Provision of tumour sample for PD-L1 evaluation*
Key exclusion criteria:
- Prior therapy
- ILD or pneumonitis requiring steroids
- Dual primary endpoints: Event-free survival (EFS) by investigator (as per RECIST v1.1) and OS§
- Secondary endpoints: mPR and pCR per blinded independent pathology review, safety
Stratification factors:
- Stage (II vs III)
- PD-L1 TPS <50% vs ≥50%*
- Histology (squamous vs non-squamous)
- Region (East Asia vs other)
Surgical details:
- KEYTRUDA arm: 92.0% (n=299/325) complete surgical resection; 78.8% lobectomies
- Placebo arm: 84.2% (n=267/317) complete surgical resection; 75.1% lobectomies
*PD-L1 was assessed at a central laboratory using PD-L1 IHC 22C3 pharmDx.1
†Platinum-containing chemotherapy consisted of cisplatin (75 mg/m2 Q3W) combined with either gemcitabine (1000 mg/m2 on Days 1 and 8 Q3W; for squamous histology only) or pemetrexed (500 mg/m2 IV Q3W; for non-squamous histology only).1
‡Radiotherapy was to be administered to participants with microscopic positive margins, gross residual disease or extracapsular nodal extension following surgery and to participants who did not undergo planned surgery for any reason other than local progression or metastatic disease.3
§One-sided alpha of 2.5% split between EFS and OS.1
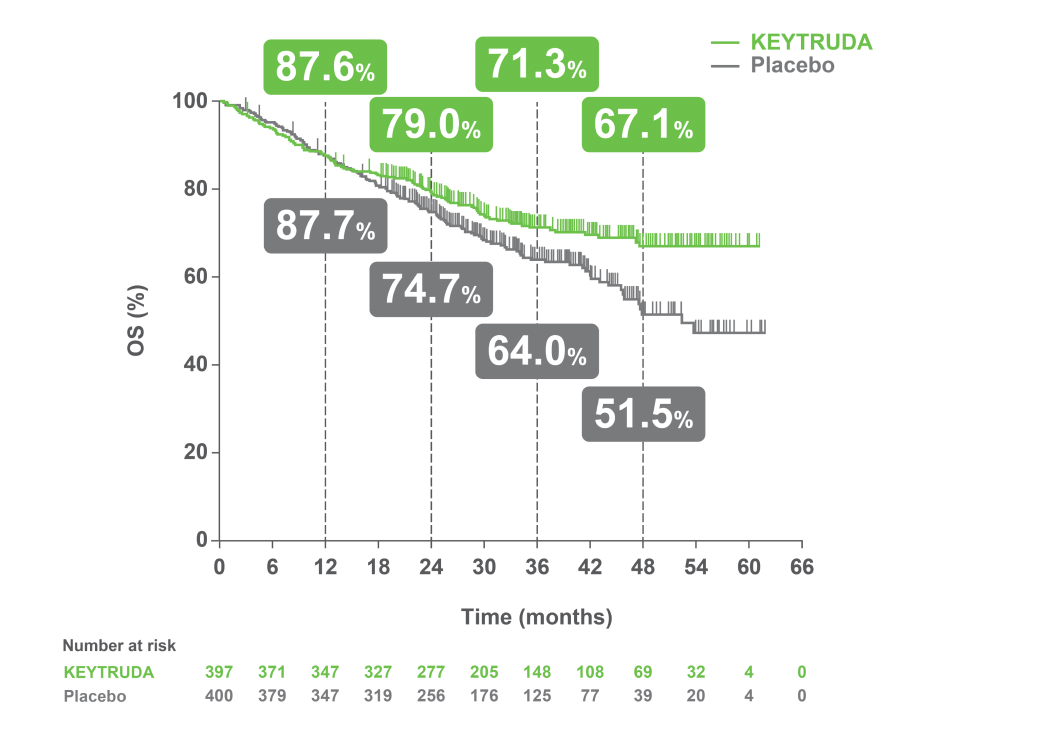
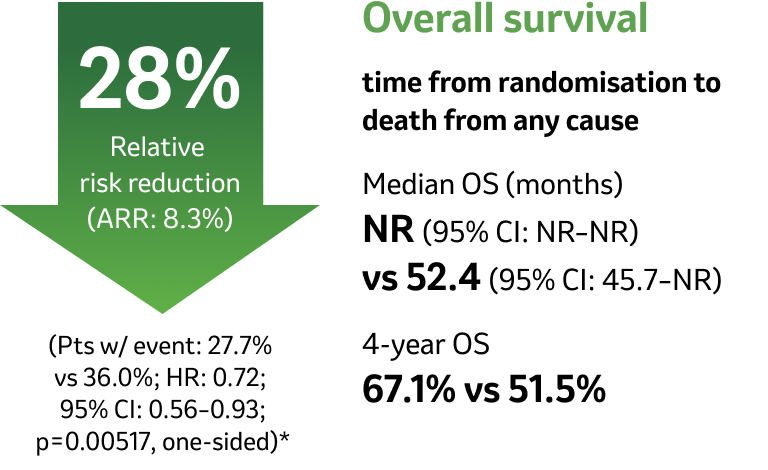
At the median follow-up of 36.6 months, perioperative KEYTRUDA demonstrated a statistically significant improvement in OS vs neoadjuvant Pt-CT and surgery alone4
KEYNOTE-671 dual primary endpoint: OS in ITT population4
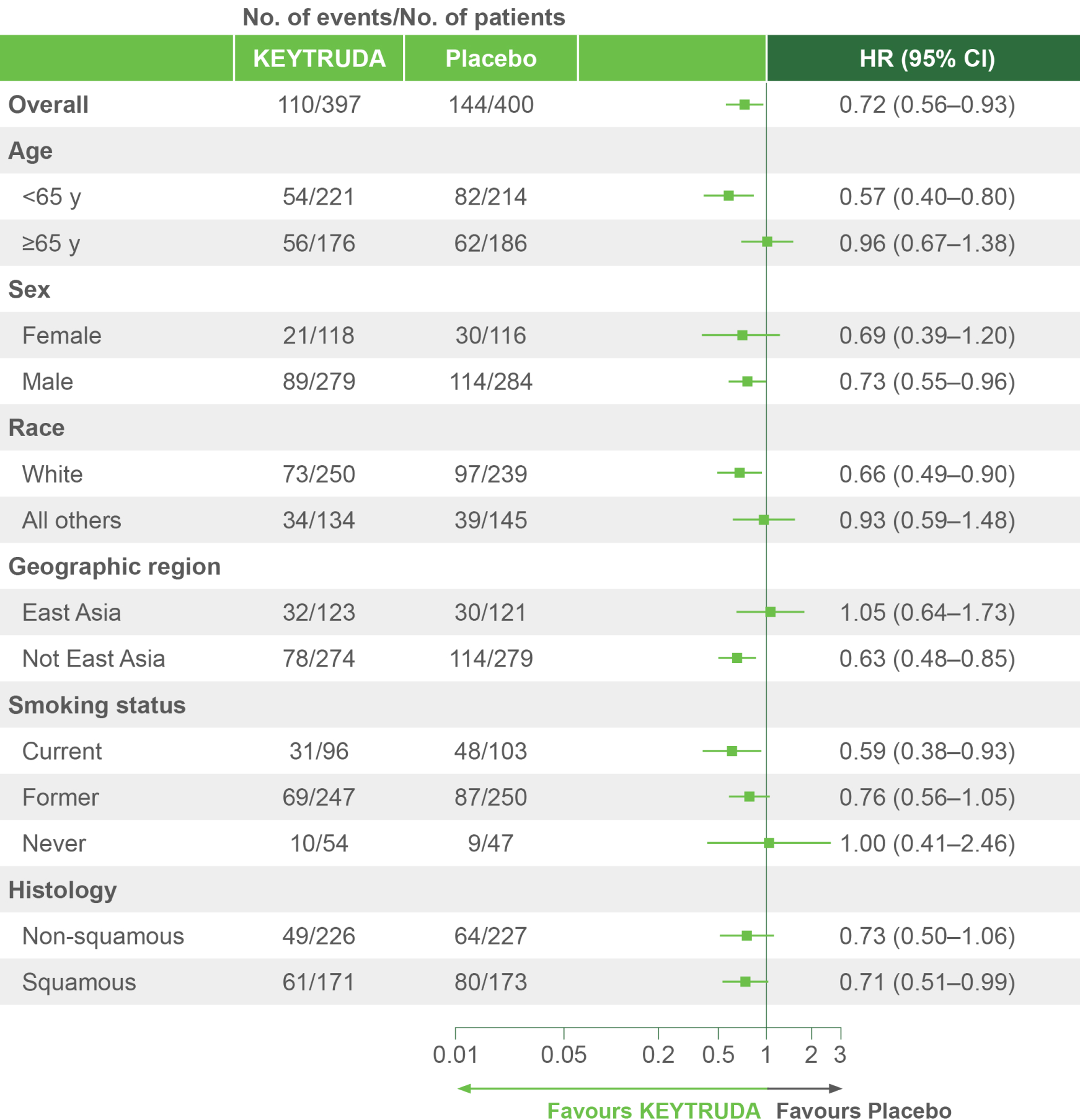
Per the prespecified analysis plan, subgroups with <30 participants are excluded from the above forest plot. Subgroups for Stage IIIA and IIIB and pN status were post hoc; all other subgroups were prespecified.
Adapted from Spicer JD, et al. 2023.4
IA2 dataset. Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
*Significance boundary at IA2, one-sided p=0.00543.4
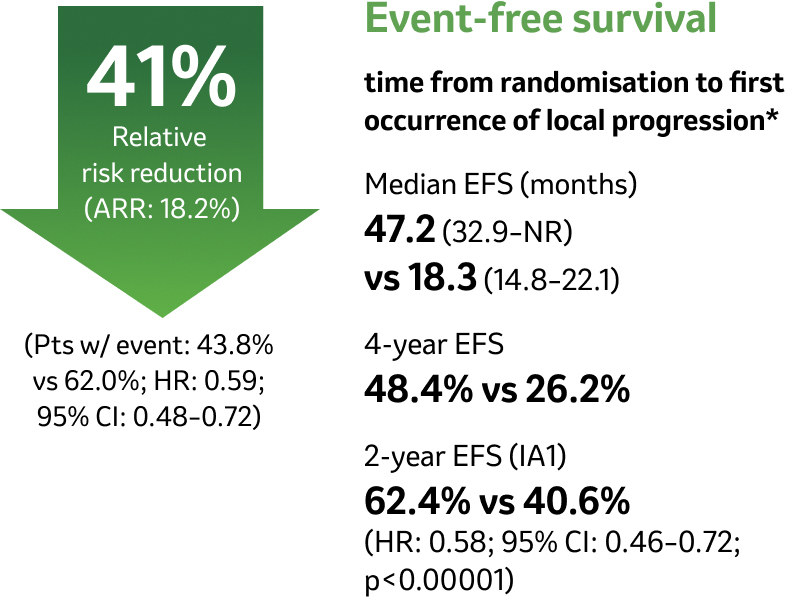
Clinically meaningful EFS benefit with KEYTRUDA – first demonstrated during IA1 – was sustained after an additional 11 months of follow-up4
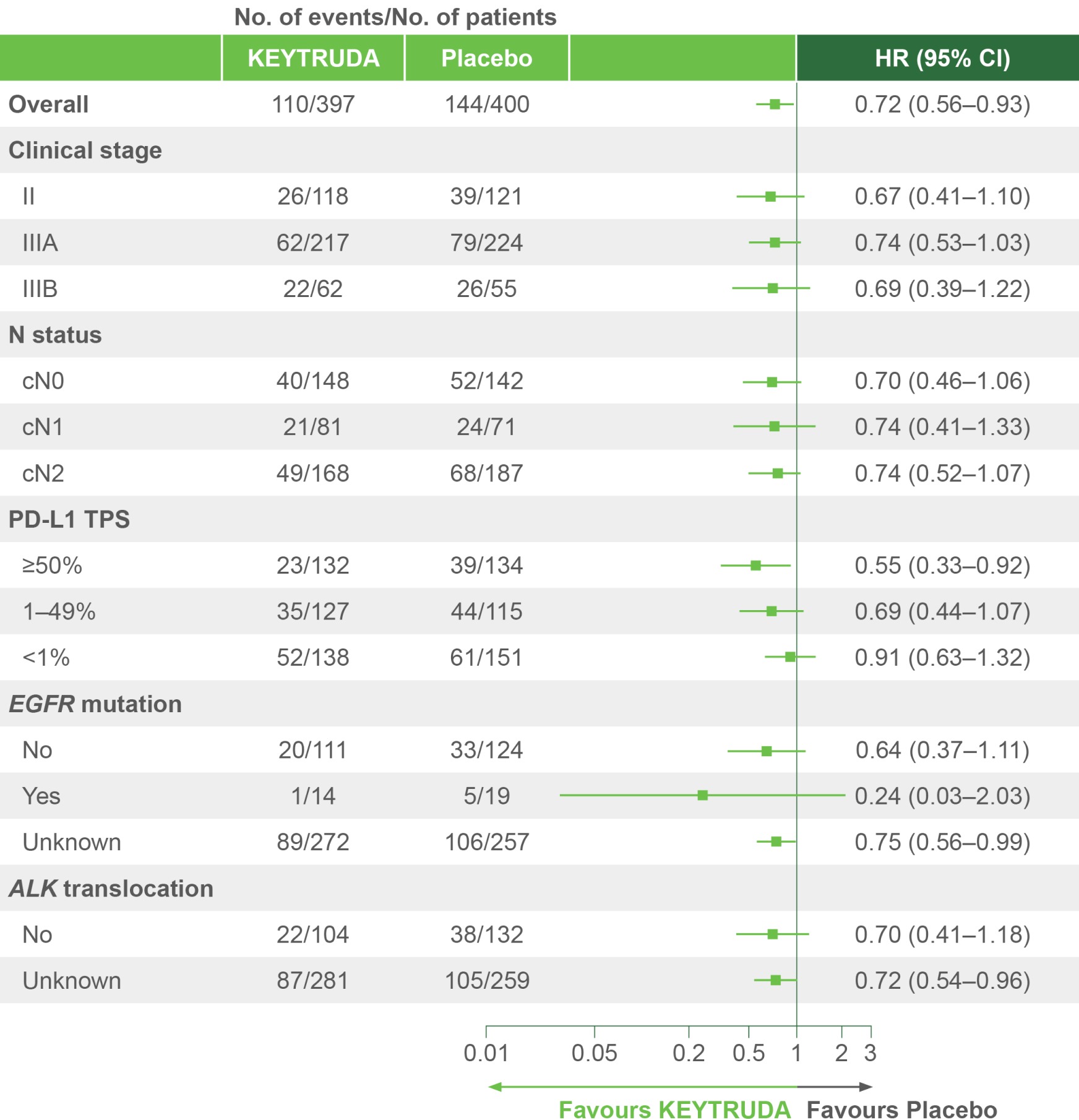
KEYNOTE-671 dual primary endpoint: EFS in ITT populations4
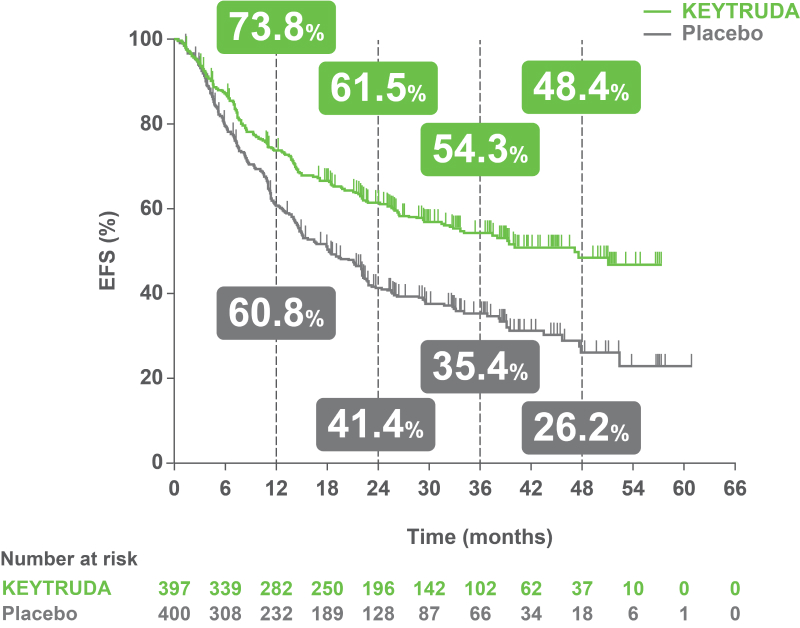
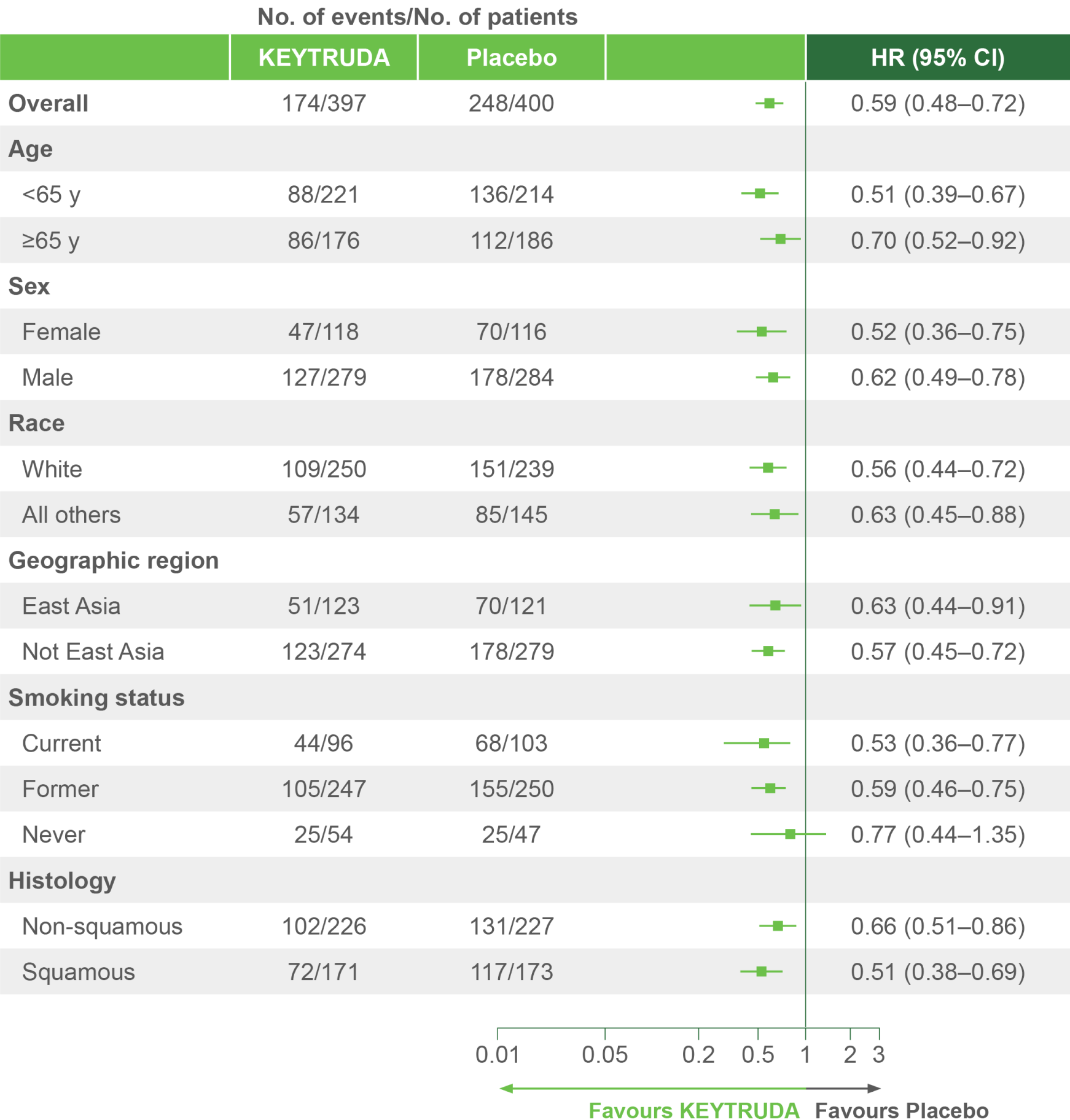
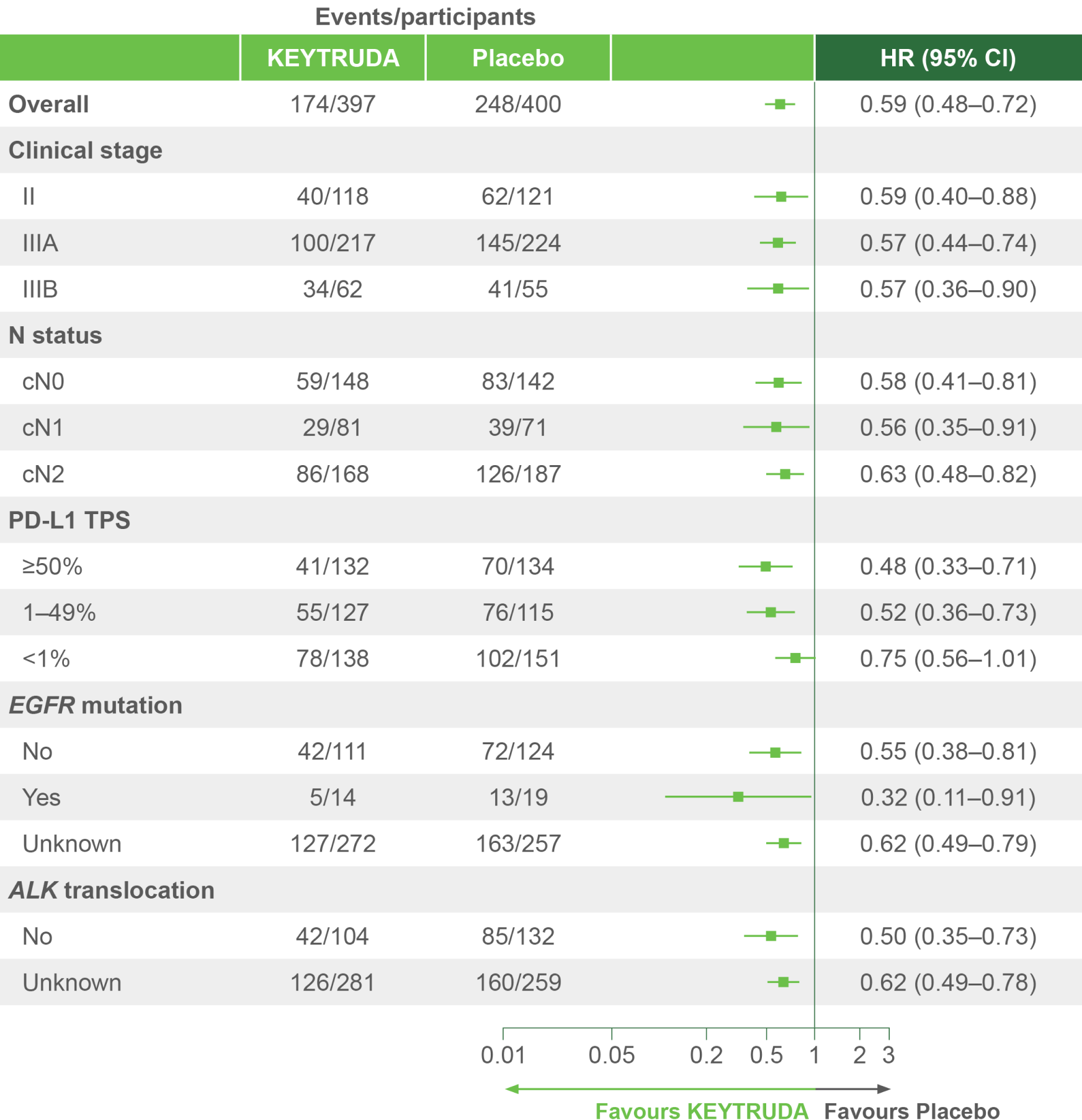
Per the prespecified analysis plan, subgroups with <30 participants are excluded from the above forest plot. Subgroups for Stage IIIA and IIIB and pN status were post hoc; all other subgroups were prespecified.
Adapted from Spicer JD, et al. 2023.4
IA2 dataset. Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
*Precluding planned surgery, unresectable tumour, progression or recurrence per RECIST v1.1 by investigator assessment, or death from any cause.4
KEYNOTE-671 safety profile (as-treated population)
The safety profile of perioperative KEYTRUDA (plus chemotherapy) was consistent with the safety profiles of the individual components, and no new safety signals were seen.1–4
Treatment-related adverse events (TRAEs), any grade4
96.7%
(383/396)
KEYTRUDA
vs
95.5%
(381/399)
Placebo
Treatment-related adverse events (TRAEs), Grade ≥3*4
45.2%
(179/396)
KEYTRUDA
vs
37.8%
(151/399)
Placebo
Treatment-related adverse events (TRAEs) that led to treatment discontinuation
13.6%
(54/396)
KEYTRUDA
vs
5.3%
(21/399)
Placebo
Treatment-related adverse events (TRAEs) that led to death
1.0%
(4/396)
KEYTRUDA
vs
0.8%
(3/399)
Placebo
IA2 dataset. Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
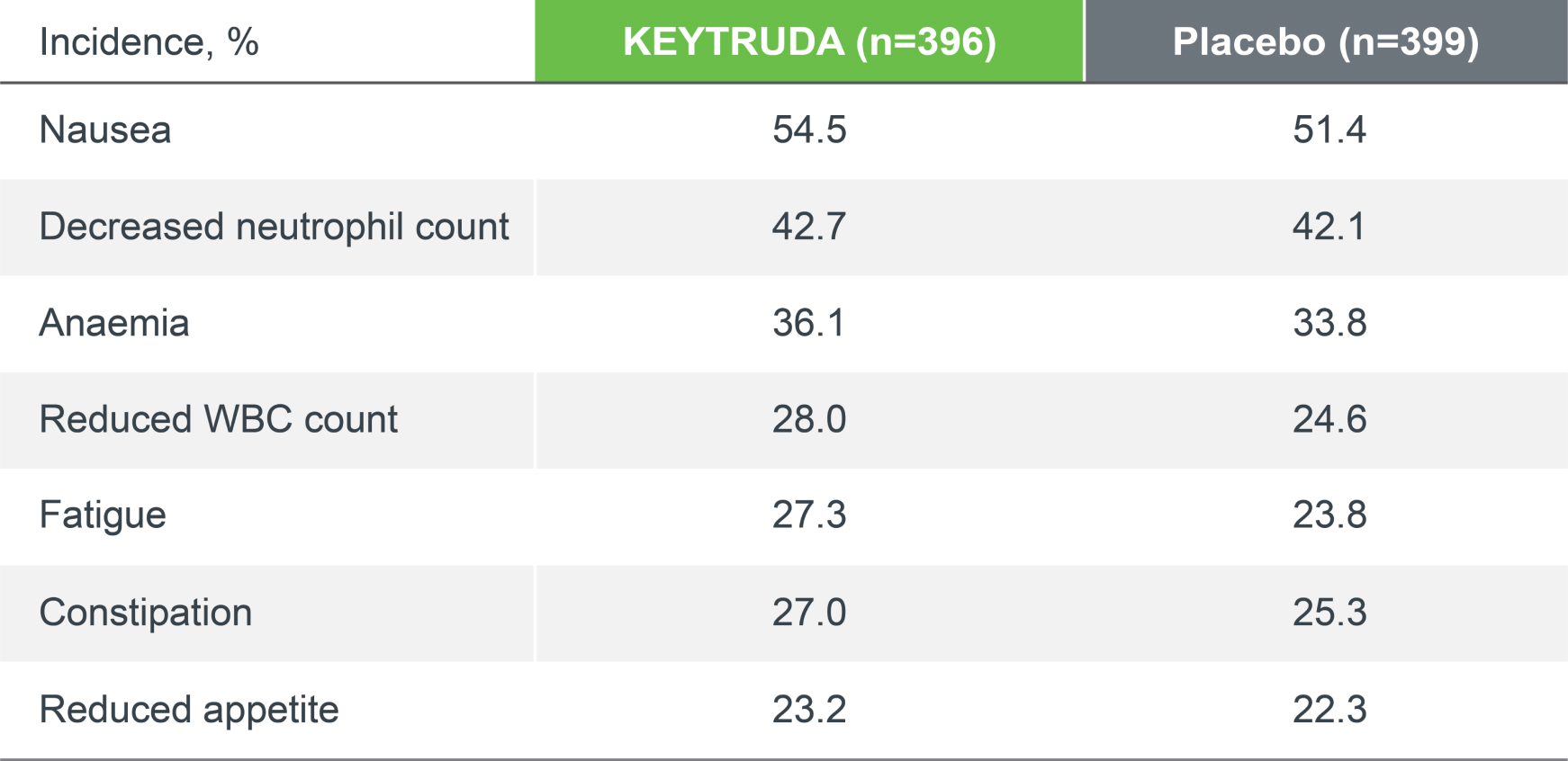
TRAEs were AEs considered by the investigator to be related to chemotherapy, KEYTRUDA and placebo.
Adapted from Spicer JD, et al. 2023.4
Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
*There were four deaths in the KEYTRUDA arm (atrial fibrillation, immune-mediated lung disease, pneumonia and sudden cardiac death). There were three deaths in the placebo arm (acute coronary syndrome, pneumonia and pulmonary haemorrhage). There have been no new treatment-related deaths during IA2 since IA1.4
KEYNOTE-671 safety profile: immune-mediated adverse events and infusion reactions
The incidence and nature of immune-mediated adverse events (IMAEs) in the KEYTRUDA arm were consistent with previous reports.4
IMAEs and infusion reactions, any grade4
26.0%
(103/396)
KEYTRUDA
vs
9.0%
(36/399)
Placebo
IMAEs and infusion reactions, Grade ≥3*4
6.6%
(26/396)
KEYTRUDA
vs
1.5%
(6/399)
Placebo
IMAEs and infusion reactions that led to treatment discontinuation
5.8%
(23/396)
KEYTRUDA
vs
0.8%
(3/399)
Placebo
IMAEs and infusion reactions that led to death
0.3%
(1/396)
KEYTRUDA
vs
0%
(0/399)
Placebo
IA2 dataset. Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
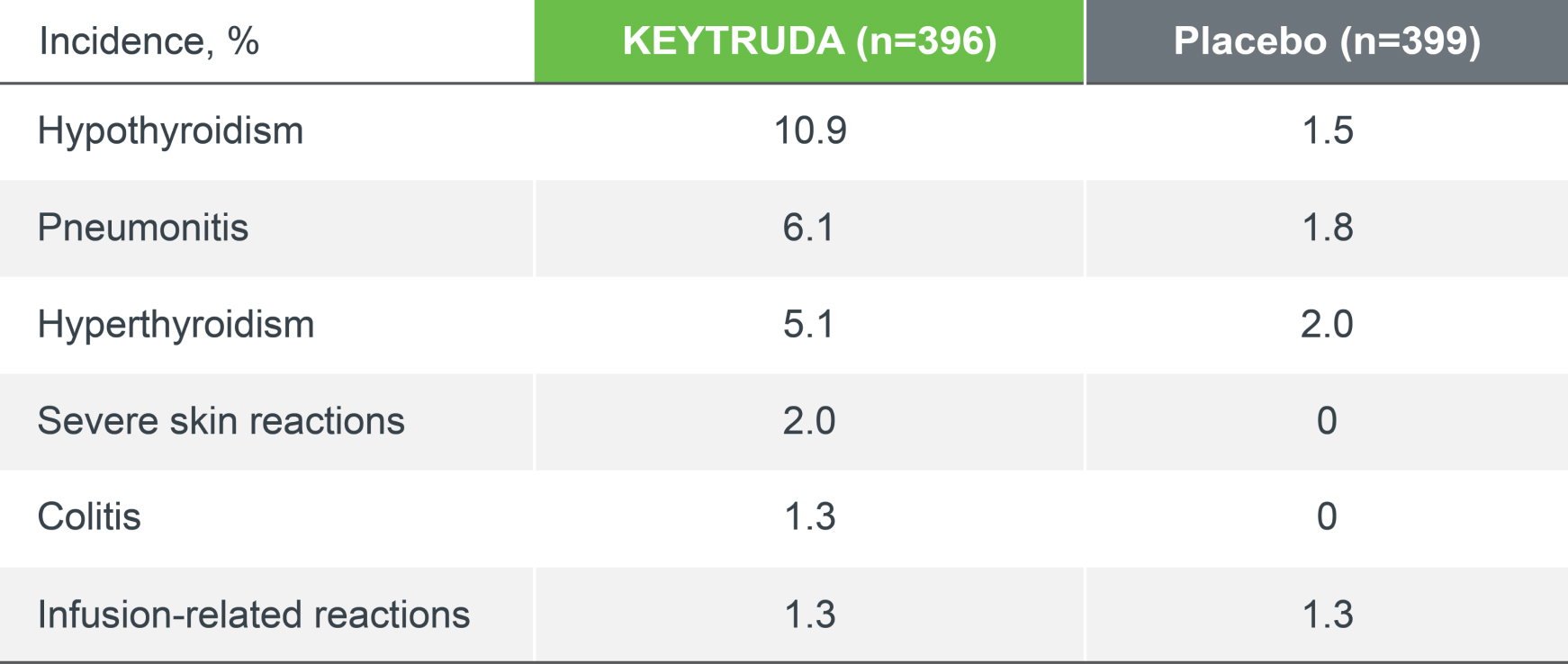
IMAEs and infusion reactions were based on a list of preferred terms intended to capture known risks of KEYTRUDA and were considered regardless of attribution to study treatment by the investigator.
Adapted from Spicer JD, et al. 2023.4
Median follow-up was 36.6 months. Data cut-off date for IA2: 10 July 2023.4
*There was one death in the KEYTRUDA arm (pneumonitis). There have been no new treatment-related deaths during IA2 since IA1.4
KEYTRUDA offers flexibility of dosing2


The 200 mg Q3W regimen has been assessed in Phase I and II registration studies across a multitude of indications of KEYTRUDA. An exposure-response evaluation, using modelling and simulation, led to the approval of the 400 mg Q6W dosing for monotherapy and combination therapy.
Chemical and physical in-use stability has been demonstrated for up to 42 days at 2˚C to 8˚C or at 23˚C to 27˚C. Protect from light. From a microbiological point of view, the product, once diluted, should be used immediately. The diluted solution must not be frozen. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 7 days at 2˚C to 8˚C, or 12 hours at room temperature, unless dilution has taken place in controlled and validated aseptic conditions. If refrigerated, the vials and/or intravenous bags must be allowed to come to room temperature prior to use.
Explore our related resources
Sign up to MSD emails
✔ Get the latest product updates
✔ Receive cancer resources
✔ Be the first to hear about our events
KEYTRUDA indications:2
- KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and platinum-based chemotherapy
- KEYTRUDA, in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment, is indicated for the treatment of resectable NSCLC at high risk of recurrence in adults
- KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥50% TPS with no EGFR- or ALK-positive tumour mutations
- KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR– or ALK-positive mutations
- KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults
- KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR– or ALK-positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA
Abbreviations
AE, adverse event; AJCC, American Joint Committee on Cancer; ALK, anaplastic lymphoma kinase; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; IA1, interim analysis 1; IA2, interim analysis 2; IHC, immunohistochemistry; ILD, interstitial lung disease; ITT, intention-to-treat; IV, intravenous; mPR, major pathological response; N0, no regional lymph node involvement1; N1, involvement of ipsilateral peribronchial and/or ipsilateral hilar lymph nodes (includes direct extension to intrapulmonary nodes)1; N2, involvement of the ipsilateral mediastinal and/or subcarinal lymph nodes1; NSCLC, non-small cell lung cancer; NR, not reached; OS, overall survival; pCR, pathological complete response; PD-L1, programmed-cell death ligand 1; pN, pathological node involvement; Q3W, every three weeks; Q6W, every six weeks; RECIST, Response Evaluation Criteria in Solid Tumours; TPS, tumour proportion score.
References
- Wakelee H, et al. N Eng J Med 2023;389:491–503.
- KEYTRUDA Summary of Product Characteristics
- Wakelee H, et al. KEYNOTE-671: Randomised, double-blind, Phase 3 study of pembrolizumab or placebo plus platinum-based chemotherapy followed by resection and pembrolizumab or placebo for early-stage NSCLC. ASCO. 2–6 June 2023. Chicago, Illinois, USA. Abstract: LBA100.
- Spicer JB, et al. Overall survival in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage NSCLC. ESMO. 20–24 October 2023. Madrid, Spain. Abstract: LBA56.
Supporting documentation
Prescribing Information [External link]
By clicking the link above you will leave the MSD Connect website and be taken to the emc PI portal website.