Training and Resources
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
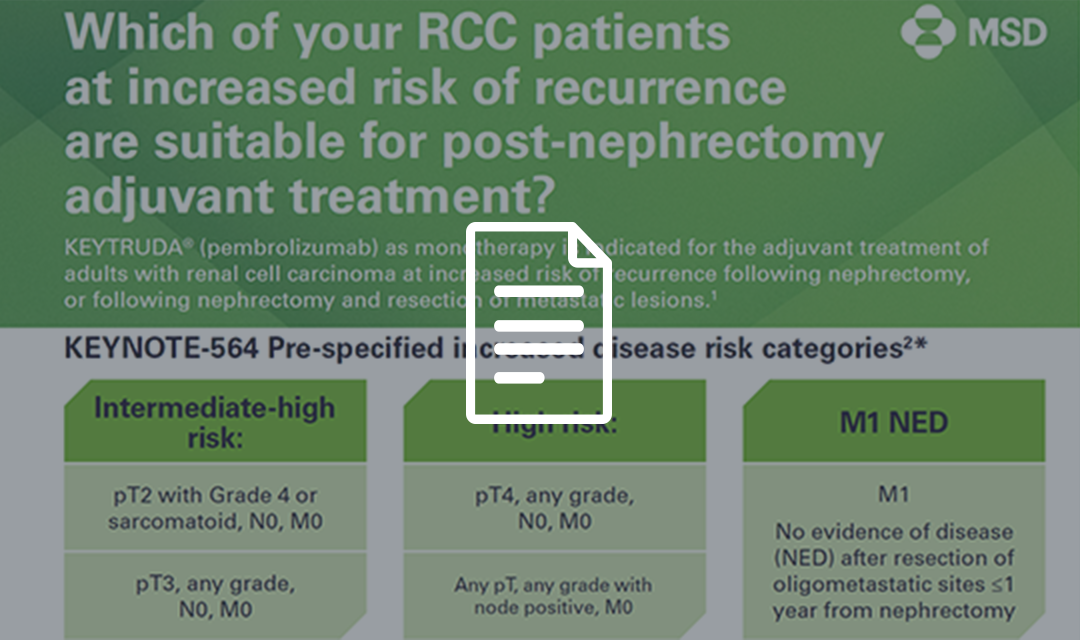
Adjuvant KEYTRUDA® (pembrolizumab) in early-stage renal cell carcinoma (RCC)

RCC Pre-Operative Considerations & Post-Operative MDT Decision Aid
Prescribing Information (Great Britain) &
Prescribing Information (Northern Ireland)
[External links]

KEYNOTE-564 slide deck
Prescribing Information (Great Britain) &
Prescribing Information (Northern Ireland)
[External links]
For more information about adjuvant KEYTRUDA in early-stage RCC:
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy or following nephrectomy and resection of metastatic lesions.1
Please refer to the Summary of Product Characteristics and risk minimisation materials before making prescribing decisions.
Reference
- KEYTRUDA Summary of Product Characteristics.
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website.